近日,美国食品和药物管理局批准类克(英利昔单抗)用于治疗6岁以上儿童的中度至重度活动期溃疡性结肠炎(UC),这些儿童对常规治疗应答不足。类克可减轻UC的症状和体征,并且能诱导和维持这些患者的临床缓解状态。
FDA药物评价和研究中心胃肠病和先天缺陷产品部主任Donna Griebel博士表示,随着类克的批准,对常规治疗应答不足的中度至重度活动性溃疡性结肠炎有了FDA批准的治疗选择。然而,该药的使用与严重风险相关,在决定开始该药治疗时,患者及家属应始终与他们的医生讨论该药物的风险和获益。
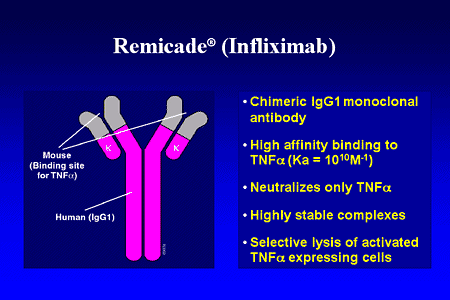
Remicade属于肿瘤坏死因子(TNF)受体阻滞剂。TNF受体阻滞剂可通过阻断TNF的活性而抑制免疫系统。除了批准用于治疗UC外,Remicade还被批准用于治疗其他自身免疫性疾病,例如成人和6岁以上儿童的克罗恩病、风湿性关节炎、强直性脊柱炎、银屑病关节炎和成人斑块状银屑病。
一项多中心、随机和开放标签研究证实了类克的安全性和有效性,纳入了60例年龄为6-17岁的中度至重度活动期UC儿童患者。所有患者对常规治疗不应答或受。
类克携带有严重感染和癌症风险的黑框警告。增加的感染风险包括肺结核和由病毒、真菌或细菌引起的感染。有使用TNF阻断剂的青少年和年轻患者报告罕见的肿瘤,包括少见和致命的肝脾T细胞淋巴瘤。
儿童应该在开始类克治疗之前接种了所有的疫苗,在接受类克治疗期间不应当接种活疫苗。类克最常见的副作用为UC恶化、上呼吸道感染、注射相关感染和头痛。
类克由宾夕法尼亚州扬森生物技术公司生产。
生物探索推荐英文原文:
FDA approves Remicade to treat ulcerative colitis in children 6 years and older
The U.S. Food and Drug Administration today approved Remicade (infliximab) to treat moderately to severely active ulcerative colitis (UC) in children 6 years and older who have had inadequate response to conventional therapy.
Remicade reduces signs and symptoms of UC and induces and maintains clinical remission in these patients.
UC is a type of inflammatory bowel disease (IBD) that affects the lining of the large intestine (colon) and rectum. Symptoms of UC include abdominal pain, diarrhea, rectal bleeding, weight loss and fever. Between 50,000 and 100,000 children in the United States have IBD; of these, 40 percent have UC.
"With the approval of Remicade, children with moderately to severely active ulcerative colitis who have not had an adequate response to conventional treatment now have an FDA-approved treatment option,” said Donna Griebel, M.D., director of the Division of Gastroenterology and Inborn Errors Products in the FDA’s Center for Drug Evaluation and Research. “However, there are serious risks associated with its use. Patients and their families should always discuss with their physician the risks and benefits of using a medication before deciding to start treatment.”
Remicade belongs to a class of drugs called tumor necrosis factor (TNF) blockers. TNF blockers suppress the immune system by blocking the activity of TNF, a substance in the body that can cause inflammation and lead to autoimmune diseases. In addition to being approved for UC, Remicade is approved for the treatment of other autoimmune diseases such as Crohn’s disease in adults and children 6 years and older, as well as rheumatoid arthritis, ankylosing spondylitis (arthritis affecting the joints in the spine and the pelvis), psoriatic arthritis (joint pain associated with psoriasis), and plaque psoriasis in adults.
The safety and efficacy of Remicade was supported by a multi-center, randomized, open-label study in 60 children ages 6 years to 17 years with moderately to severely active UC. All had failed to respond to or tolerate conventional treatment.
Remicade carries a Boxed Warning for risk of serious infections and cancer. Increased risks of infections include tuberculosis and infections caused by viruses, fungi or bacteria. There have been cases of unusual cancers reported in adolescent and young adult patients using TNF-blocking agents, including a rare and fatal type of cancer called Hepatosplenic T-cell Lymphoma.
Children should have all of their vaccines brought up to date before starting treatment with Remicade and should not receive live vaccines while taking Remicade. The most common side effects of Remicade are worsening of UC, upper respiratory infections, infusion-related reactions, and headache.
Remicade is manufactured by Janssen Biotech Inc. in Malvern, Pa.







