You may very well have prepared a nearly perfect gel, and would have a difficult time improving upon the product. If that is so, then by all means gloat about it! If you didn't do such a hot job don't despair. All good scientists learn from their mistakes, in fact, without making mistakes most of us wouldn't learn much at all!
The "Hall of Shame" presents examples of some of the worst gels students (and instructor) have run in past labs, with an example or two from a research lab. They represent many of the ways one can mess up a gel (but not all of them - we're still finding new ways!). See what features of your own gel(s) were unsatisfactory - or at least less than perfect - and use the illustrations to figure out what you might do to improve your technique.
To critique your own work identify your symptoms and use the gallery to select appropriate example(s). Each example is linked to a full sized image with suggessted explanations for the symptoms. From this "hall of shame" you should be able to determine what can be done to correct the problem(s). If you wish you may browse by descriptions of symptoms, listed below the gallery.
Symptoms 1—Smears
Smeared gels – example 1
Smearing can have a variety of causes, but most commonly it is due to an unevenly poured acrylamide mixture or due to gross overloading of protein.

In this example the gel was not properly poured, so that the lower half had begun to polymerize before the upper part was poured. The first gel mix began to polymerize too quickly. Rather than prepare a new gel cassette, the students simply stopped pouring, prepared a new mix, and poured it on top of the old. Obviously, the bond wasn't particularly good.
Smeared gels – example 2
In this example a lot of protein was loaded in each of the wells, all the way across. Most of the lanes can still be interpreted, but smearing is particularly evident in lanes 3 and 4. Those lanes contained primarily one protein (hemoglobin subunit), as did lanes 9 and 10. However, the group that loaded 3 and 4 underestimated the protein concentrations in their red cell lysate and red cell cytoplasm fractions. Those fractions contained so much protein that samples had to be diluted prior to preparing a protein assay tube. The students forgot to take the dilution factor into consideration when determining protein concentration.
The capacity of a mini-gel for a mixed protein sample is 20 to 40 micrograms/well, depending on the resolution needed and number individual polypeptides in the mix. However, if a pure protein is loaded, one single band will contain all 20-40 micrograms, and the result is a mess.

Smeared gels – example 3
Here is another example of gross overloading, and it appears these students made the same mistake as in example 2. It looks as though there was some inconsistency in the gel also.

Smeared gels – example 4
Smearing can be a normal consequence of running membrane-associated proteins with a high lipid content in a discontinuous gel. In discontinuous PAGE, sample proteins are super-concentrated by a combination of two factors. First, the rate of migration is suddenly slowed as the sample moves from a non-restrictive stacking gel to a separating gel that restricts movement. Second (and more important), a pH change affects the electrophoretic mobility of proteins in the sample, causing the sample to check up abruptly. A sample of a half cm or so in height becomes compressed into a layer of bands a few micrometers thick, and the local concentration of protein goes up considerably.
Membrane-associated proteins tend to precipate at lower concentrations, thus lanes containing such proteins often have a dark band of precipitated proteins at the very top. As electrophoresis proceeds the precipates re-dissolve and enter the gel continuously, thus forming a continuous dark background of unresolved polypeptides. Many of the membrane samples shown on these gels will show that characteristic.
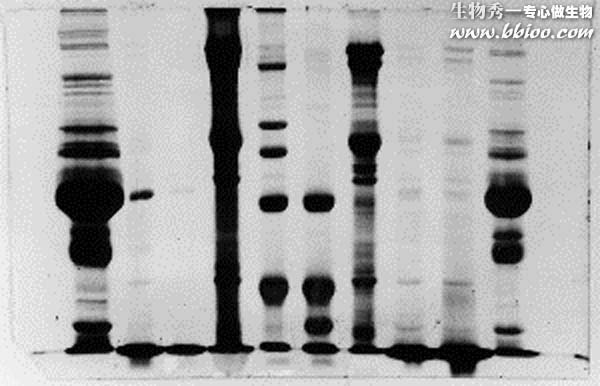
The resolved bands in the membrane protein lanes (4 and 7) appear to consist of similar amounts of protein, however in lane 4 the capacity of the gel for at least some of the membrane proteins was exceeded, resulting in a dark smear.
Symptoms 2—Streaks
Streaks – example 1
This one isn't so bad. The lane with the streaks (left of lane 5) is quite usable, in fact. The streaks came about because a portion of the more concentrated proteins in the membrane fraction precipated during the stacking process, then returned to solution after a fairly short time. Because of slight imperfections in the separating gel surface (it may have been allowed to dry a little before adding the stacking gel), the protein concentration was not uniform across the lane, and streaks rather than a uniform darker background are the result.
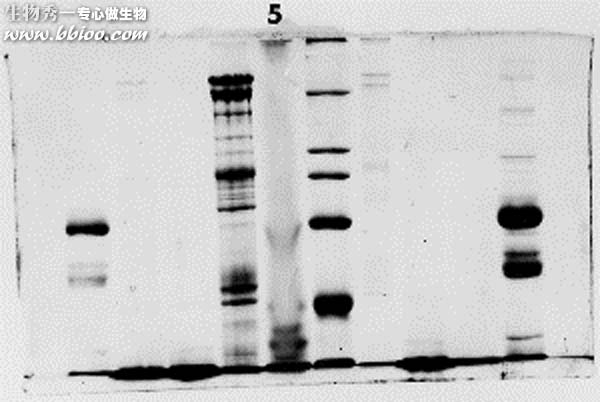
Streaks – example 2
The fourth lane is streaked due to the effect described above. The electrical field was affected by the non-uniform concentration in the fourth lane (the dark one with streaks),, causing a narrowing of the bands. Note that the dye front was lost in this gel - it was allowed to run too long.
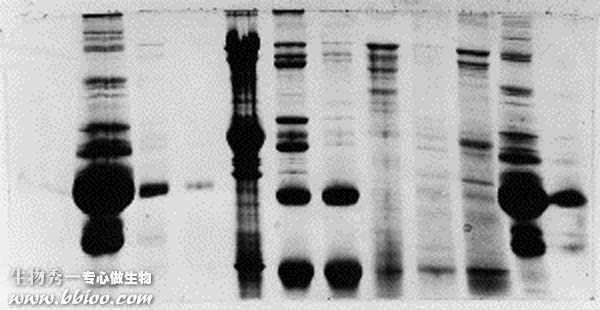
Streaks – example 3
This one kind of stinks. Evidently, this group did not use an overlay after pouring the first gel layer. The result was a separating gel with the very rough top that you see here. As samples were stacked they were disproportionately concentrated in the 'valleys,' where the proteins precipitated. The precipitates re-dissolved gradually during the run, forming streaks instead of resolving into discrete bands.
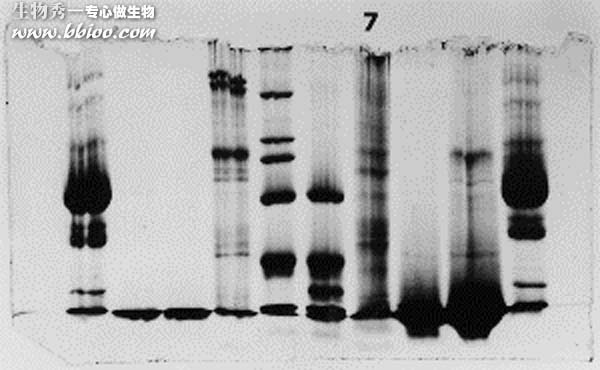
Symptoms 3—Bands too light
Light bands – example 1
It looks like this problem was caused by badly formed wells and/or careless loading of sample onto the gel. The background is dark and uneven, suggesting that protein was in the running buffer itself. Most likely the apparatus was jarred and much of the samples was slopped out of the wells. The spilled sample would not resolve into bands,since it continuously entered the gel from the upper buffer compartment.
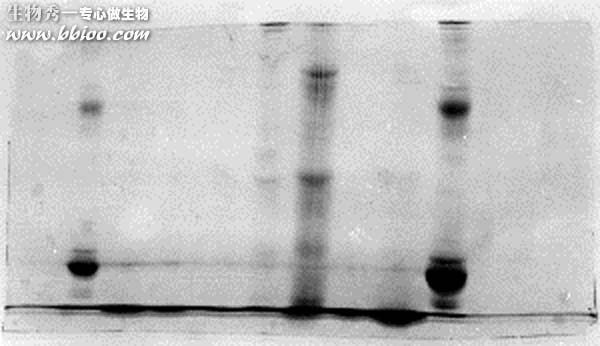
Note the presence of a horizontal line, continuous along several lanes (arrow). That would suggest that the wells were too shallow, and some material spilled into adjacent wells.







