据美国物理学家组织网11月14日(北京时间)报道,美国科学家研制出一种名为KG5的新药,能通过依附于酶RAF之上并改变其结构来抑制几乎所有肿瘤细胞的分裂,从而破坏增生能力最强的肿瘤。科学家们表示:“这个非同寻常的新成果能真正挑战现有的医学教条。”相关研究发表在11月13日出版的《自然·医学》网络版上。
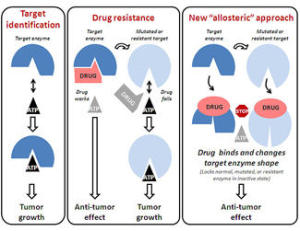
目前攻击RAF等酶的抗癌药物一般都被设计成同酶的活性部位相互作用,但这样常常会使其缺乏针对性。为避免现有药物的限制,摩尔斯癌症研究中心转化医学研究小组副主任、病理学教授戴维·切瑞西领导的研究团队研制出新型RAF抑制剂KG5。它可以改变酶的结构让酶失去活性。切瑞西表示:“尽管RAF已是科学家的‘老朋友’,但它在对细胞增生和肿瘤生长非常关键的细胞分裂中的作用仍不为人所知悉。通过设计出一种能改变RAF形状的新型药物,我们能揭示RAF在很多肿瘤中未被发现的作用。”
测试结果表明,KG5能在不断增生的细胞中挑选出RAF,但会忽视正常的或沉默的细胞。而且在受到其影响的肿瘤细胞中,RAF不能同细胞有丝分裂器相互联系来引导细胞分裂,从而致使肿瘤细胞凋亡。另外,KG5也能采用同样的方式有效地干预血管新生,从而干预肿瘤的生长以及肿瘤的转移。切瑞西指出:“这个非同寻常的发现能真正挑战现有的教条。通过设计出‘绕过’酶的活性部位的新药,我们找到了破坏肿瘤生长的新方法。从本质上而言,我们正在采用一种全新的方式攻击一种重要的酶。”
科学家们对癌细胞系、动物模型以及从癌症病人身上提取出来的活组织进行了测试,KG5都产生了同样的结果。(生物探索)
相关英文论文摘要:
A MEK-independent role for CRAF in mitosis and tumor progression
RAF kinases regulate cell proliferation and survival and can be dysregulated in tumors1, 2. The role of RAF in cell proliferation has been linked to its ability to activate mitogen-activated protein kinase kinase 1 (MEK) and mitogen-activated protein kinase 1 (ERK). Here we identify a MEK-independent role for RAF in tumor growth. Specifically, in mitotic cells, CRAF becomes phosphorylated on Ser338 and localizes to the mitotic spindle of proliferating tumor cells in vitro as well as in murine tumor models and in biopsies from individuals with cancer. Treatment of tumors with allosteric inhibitors, but not ATP-competitive RAF inhibitors, prevents CRAF phosphorylation on Ser338 and localization to the mitotic spindle and causes cell-cycle arrest at prometaphase. Furthermore, we identify phospho-Ser338 CRAF as a potential biomarker for tumor progression and a surrogate marker for allosteric RAF blockade. Mechanistically, CRAF, but not BRAF, associates with Aurora kinase A (Aurora-A) and Polo-like kinase 1 (Plk1) at the centrosomes and spindle poles during G2/M. Indeed, allosteric or genetic inhibition of phospho-Ser338 CRAF impairs Plk1 activation and accumulation at the kinetochores, causing prometaphase arrest, whereas a phospho-mimetic Ser338D CRAF mutant potentiates Plk1 activation, mitosis and tumor progression in mice. These findings show a previously undefined role for RAF in tumor progression beyond the RAF-MEK-ERK paradigm, opening new avenues for targeting RAF in cancer.
英文论文链接:https://www.nature.com/nm/journal/vaop/ncurrent/full/nm.2464.html







