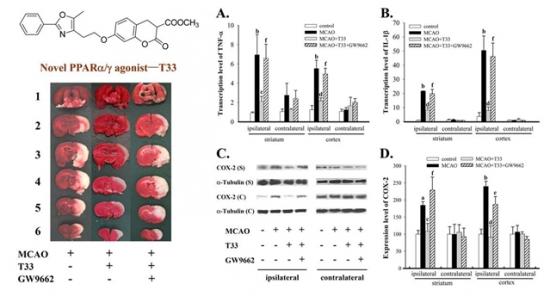
T33对大脑中动脉栓塞诱发脑梗死的保护作用和抗炎症作用。左上角为T33的结构式,左下角为脑切片TTC染色图,T33能明显减少缺血诱发的大脑梗死,而用PPARg拮抗剂—GW9662能部分阻断T33的保护作用。图中A,B分别代表TNF-a和IL-1b的基因转录水平;C,D分别代表COX-2的蛋白电泳代表性图片和统计数据。
缺血性脑中风,是一种突发性的脑血液循环障碍性疾病。溶栓药组织纤溶酶原激活剂—tPA是目前唯一被美国FDA批准应用于治疗缺血性脑中风的临床药物,但是其发挥药效的主要原理是通过溶解血栓使血流再灌恢复脑部供血供氧,并不能修复以及阻止受损脑区病变,因此迫切需要寻找新型的具有神经保护作用的抗缺血性脑中风类药物。
中国科学院上海药物研究所新药研究国家重点实验室章海燕课题组与杨玉社课题组的科研人员通过合作,发现新型非TZD类PPARγ/α双激动剂——T33可以显著减少急、慢性体外缺血模型和急性脑缺血动物模型导致的炎症因子过度表达,并且对脑缺血诱发皮层和纹状体脑组织梗死具有明显的改善作用。
T33的抗炎作用可能是通过干预NFκB信号通路以及p38信号通路发挥的,并且提示其PPAR激动作用在很大程度上参与了上述抗炎及神经保护作用。该成果进一步验证了PPAR作为中风药物靶标的可行性,并且为研发对抗缺血性中风的新型药物提供了重要的线索和理论依据。
该项目得到了国家重大新药创制专项课题、国家自然科学基金项目的支持。研究成果于2011年9月发表在《中国药理学报》
T33对大脑中动脉栓塞诱发脑梗死的保护作用和抗炎症作用。左上角为T33的结构式,左下角为脑切片TTC染色图,T33能明显减少缺血诱发的大脑梗死,而用PPARg拮抗剂—GW9662能部分阻断T33的保护作用。图中A,B分别代表TNF-a和IL-1b的基因转录水平;C,D分别代表COX-2的蛋白电泳代表性图片和统计数据。
缺血性脑中风,是一种突发性的脑血液循环障碍性疾病。溶栓药组织纤溶酶原激活剂—tPA是目前唯一被美国FDA批准应用于治疗缺血性脑中风的临床药物,但是其发挥药效的主要原理是通过溶解血栓使血流再灌恢复脑部供血供氧,并不能修复以及阻止受损脑区病变,因此迫切需要寻找新型的具有神经保护作用的抗缺血性脑中风类药物。
中国科学院上海药物研究所新药研究国家重点实验室章海燕课题组与杨玉社课题组的科研人员通过合作,发现新型非TZD类PPARγ/α双激动剂——T33可以显著减少急、慢性体外缺血模型和急性脑缺血动物模型导致的炎症因子过度表达,并且对脑缺血诱发皮层和纹状体脑组织梗死具有明显的改善作用。
T33的抗炎作用可能是通过干预NFκB信号通路以及p38信号通路发挥的,并且提示其PPAR激动作用在很大程度上参与了上述抗炎及神经保护作用。该成果进一步验证了PPAR作为中风药物靶标的可行性,并且为研发对抗缺血性中风的新型药物提供了重要的线索和理论依据。
该项目得到了国家重大新药创制专项课题、国家自然科学基金项目的支持。研究成果于2011年9月发表在《中国药理学报》
相关英文论文摘要:
T33, a novel peroxisome proliferator-activated receptor
|[gamma]|/|[alpha]| agonist, exerts neuroprotective action via its anti-inflammatory activities
Aim: To examine the neuroprotective effects of T33, a peroxisome proliferator-activated receptor gamma/alpha (PPARγ/α) agonist, in acute ischemic models in vitro and in vivo. Methods: Primary astrocytes subjected to oxygen-glucose deprivation/reperfusion (O/R) and BV-2 cells subjected to hypoxia were used as a model simulating the ischemic core and penumbra, respectively. The mRNA levels of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) were measured using qPCR. The levels of TNF-α secreted by BV-2 cells were measured using ELISA. Protein levels of cyclooxygenase-2 (COX-2), p65, phosphorylated I-κBα/I-κBα, phosphorylated I-κB kinase (pIKK), phosphorylated eukaryote initiation factor 2α (p-eIF-2α)/eIF-2α and p-p38/p38 were detected using Western blot. PPARγ activity was measured using EMSA. The neuroprotection in vivo was examined in rat middle cerebral artery occlusion (MCAO) model with neurological scoring and TTC staining. Results: Addition of T33 (0.5 μmol/L) increased the level of I-κBα protein in primary astrocytes subjected to O/R, which was due to promoting protein synthesis without affecting degradation. In primary astrocytes subjected to O/R, addition of T33 amplified I-κBα gene transcription and mRNA translation, thus suppressing the nuclear factor-kappa B (NF-κB) pathway and reducing inflammatory mediators (TNF-α, IL-1β, and COX-2). In BV-2 cells subjected to hypoxia, T33 (0.5 μmol/L) reduced TNF-α, COX-2, and p-P38 production, which was antagonized by pre-administration of the specific PPARγ antagonist GW9662 (30 μmol/L). T33 (2 mg/kg, ip) attenuated MCAO-induced inflammatory responses and brain infarction, which was antagonized by pre-administered GW9662 (4 mg/kg, ip). Conclusion: T33 exerted anti-inflammatory effects in the ischemic core and penumbra via PPARγ activation, which contributed to its neuroprotective action.
英文论文链接:https://www.nature.com/aps/journal/v32/n9/full/aps201169a.html







