瑞士霍夫曼·罗氏的研究员Susanne Ostrowitzki博士及其同事报告的一项随机、安慰剂对照、双盲研究显示,一种直接作用于β-淀粉样蛋白肽的单克隆抗体——gantenerumab,可剂量依赖性地消除阿尔茨海默病患者脑中的淀粉样蛋白。该研究发表在《神经病学文献》10月10日在线版上。
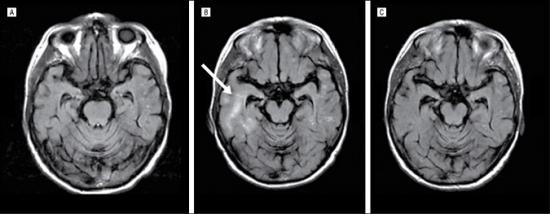
如图所示,A为对照组,B为治疗中,C为治疗后,从这组MRI扫描图可以看出,淀粉样蛋白被清除。
Gantenerumab是一种完全人源化单克隆抗体,可与β-淀粉样蛋白斑块特异性结合。这项研究招募了轻至中度阿尔茨海默病患者,对静脉注射该抗体2~7次和注射安慰剂进行了比较。每4周注射1次,每次注射60或200 mg。本次报告的是其中16例患者的结果。在基线时和治疗期间,通过正电子发射段断层扫描(PET)检测了这些患者脑中β-淀粉样蛋白沉积部位和数量。
治疗结束时,安慰剂组4例患者的PET标化吸收值比率(SUVR)平均比基线时增加了21%,60 mg gantenerumab组6例患者的PET- SUVR平均增加5%,200 mg gantenerumab组6例患者则平均下降了15%。这种组间差异在脑中各个区域均存在,唯一的例外是脑桥,而后者已知是淀粉样蛋白较少沉积的区域。在2~7次注射后,gantenerumab对淀粉样蛋白沉积的效果迅速显现。
研究者未能观察到与淀粉样蛋白改变相一致的认知指标变化,但他们认为这与样本量太小和治疗时间太短有关。
该研究团队对新采集的人类胶质细胞开展实验时发现,gantenerumab降低淀粉样蛋白水平的作用可能与细胞吞噬有关。这一发现提示,gantenerumab可在不显著改变血管通透性(通过炎症或阻断β-淀粉样蛋白清除通路)的情况下,即产生降低淀粉样蛋白水平的作用。
虽然上述结果令人鼓舞,但研究者承认该研究规模过小,且减少淀粉样蛋白的后续临床意义仍属未知,“尚不明确脑内淀粉样蛋白水平下降是否能转化为临床收益”。目前正在开展一项2期临床试验,以了解前驱期阿尔茨海默病患者接受gantenerumab治疗是否有临床收益。
Ostrowitzki博士及部分合著者是霍夫曼-罗氏的雇员,该公司资助了这项研究。另有2名合著者是通用电气医疗的雇员。
相关英文论文摘要:
Mechanism of Amyloid Removal in Patients With Alzheimer Disease Treated With Gantenerumab
Background
Gantenerumab is a fully human anti-Aβ monoclonal antibody in clinical development for the treatment of Alzheimer disease (AD).
Objectives To investigate whether treatment with gantenerumab leads to a measurable reduction in the level of Aβ amyloid in the brain and to elucidate the mechanism of amyloid reduction.
Design
A multicenter, randomized, double-blind, placebo-controlled, ascending-dose positron emission tomographic study. Additionally, ex vivo studies of human brain slices from an independent sample of patients who had AD were performed.
Setting
Three university medical centers.
Patients
Patients with mild-to-moderate AD.
Intervention
Two consecutive cohorts of patients received 2 to 7 infusions of intravenous gantenerumab (60 or 200 mg) or placebo every 4 weeks. Brain slices from patients who had AD were coincubated with gantenerumab at increasing concentrations and with human microglial cells.
Main Outcome Measures Percent change in the ratio of regional carbon 11–labeled Pittsburgh Compound B retention in vivo and semiquantitative assessment of gantenerumab-induced phagocytosis ex vivo.
Results
Sixteen patients with end-of-treatment positron emission tomographic scans were included in the analysis. The mean (95% CI) percent change from baseline difference relative to placebo (n = 4) in cortical brain amyloid level was –15.6% (95% CI, –42.7 to 11.6) for the 60-mg group (n = 6) and –35.7% (95% CI, –63.5 to –7.9) for the 200-mg group (n = 6). Two patients in the 200-mg group showed transient and focal areas of inflammation or vasogenic edema on magnetic resonance imaging scans at sites with the highest level of amyloid reduction. Gantenerumab induced phagocytosis of human amyloid in a dose-dependent manner ex vivo.
Conclusion
Gantenerumab treatment resulted in a dose-dependent reduction in brain amyloid level, possibly through an effector cell–mediated mechanism of action.
英文论文链接:https://archneur.ama-assn.org/cgi/content/full/archneurol.2011.1538







