纤维连接蛋白出现在伤口上
科学家们发现了细胞检测组织损伤并相应修改它们的修复特征的方式。这项10月6日发表在《Developmental Cell》杂志上的发现为相关疾病或进行外科手术的患者改善组织修复提供了新的机会。
这项研究由惠康基金会(Wellcome Trust)资助,布里斯托尔大学的生物化学家主导。他们检测了受损组织细胞中的信号程序,鉴定出了负责激活有效修复的细胞机制。
在健康的成年人中,大多数的组织细胞在未受到创伤时是休眠的,一旦受到创伤,它们即感受到细胞环境中的变化。受损血管出现血浆渗漏并导致成纤维细胞迁移进入受损组织,与伤口接触后,通过沉淀诸如提供结构支持的胶原蛋白来塞住缝隙。
论文的主要作者、该大学生物化学学院的研究员Mark Bass博士说:“这些过程的每一步都需要细胞黏结的运转,困难的是要确定细胞是如何检测到组织损伤并相应地修改它们的黏着特征的。”
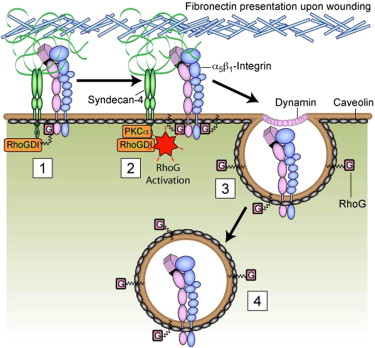
通过使用原子力显微镜,该研究小组可以确定分子传感器多配体聚糖4触发对黏性分子的摄入及重新分配的方式。这种新的信号途径导致成纤维细胞与角化细胞的迁移以响应变化的组织结构,并听从形成皮肤的基质纤维的调配。此类指向受损信号的线性迁移使得细胞快速到达伤口,这要比细胞随机搜索组织伤口要有效的多,从而形成有效的治疗反应。
Bass博士补充说道:“我们发现这种信号流对于有效治疗非常必要,这为改善患者的组织修复提供的相当的机会。”
惠康基金会资助了该项研究,Mark Bass博士将研究结果以“A syndecan-4 hair trigger initiates wound healing through caveolin- and RhoG-regulated integrin endocytosis”为题发表在《Developmental Cell》杂志上。(生物探索william译)
相关英文论文摘要:
A syndecan-4 hair trigger initiates wound healing through caveolin- and RhoG-regulated integrin endocytosis
Cell migration during wound healing requires adhesion receptor turnover to enable the formation and disassembly of cell-extracellular matrix contacts. Although recent advances have improved our understanding of integrin trafficking pathways, it is not known how extracellular ligand engagement controls receptor dynamics. Using atomic force microscopy, we have measured cell avidity for fibronectin and defined a mechanism for the outside-in regulation of α5β1-integrin. Surprisingly, adhesive strength was attenuated by the syndecan-4-binding domain of fibronectin due to a rapid triggering of α5β1-integrin endocytosis. Association of syndecan-4 with PKCα was found to trigger RhoG activation and subsequent dynamin- and caveolin-dependent integrin uptake. Like disruption of syndecan-4 or caveolin, gene disruption of RhoG in mice was found to retard closure of dermal wounds due to a migration defect of the fibroblasts and keratinocytes of RhoG null mice. Thus, this syndecan-4-regulated integrin endocytic pathway appears to play a key role in tissue repair.







