干细胞非常易变,它会成什么器官或组织一直让科学家琢磨不定。据美国物理学家组织网8月28日报道,最近,在美国化学学会第242届国际学术研讨会暨展览会上,科学家们汇报了一种先进的细胞培养表面,有助于科学家控制干细胞的发育方向。该研究为再生医学领域提供了一种先进方法,以此培养的器官和组织可用于移植和治疗疾病。
多能性人类胚胎干细胞是从胚胎衍生而来的细胞,具有发育成上百种细胞的可能。培养系统具有某种“不确定性”,不同批次的小鼠细胞可能出现多种变化,而控制它们的分化方向是干细胞医疗应用研究中的一大障碍。以前的方法是添加调控生长的物质,但这可能会给细胞带来无法预料的伤害。
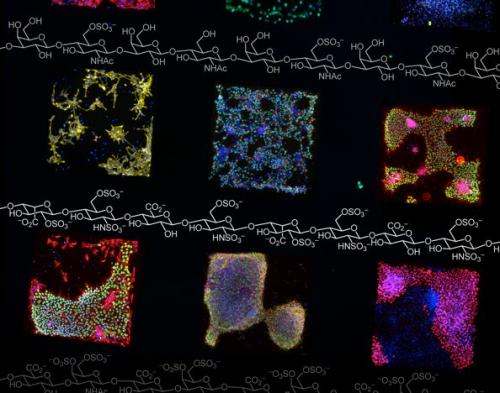
Cells growing on surfaces with different materials on them are shown in colored squares.
美国威斯康辛大学化学系的劳拉•L•基斯林博士在报告会上表示,干细胞在再生医学、新药开发、生物医疗前沿中都有很大潜力,但要开发这种潜力必须克服两个难题:第一,找到能在实验室培养繁殖人类干细胞的方法;第二,控制干细胞按需分化,生长为心脏、大脑等任意类型的细胞。新方法在解决这些问题方面取得了良好效果。
基斯林和同事人工合成出一种纯化学培养基面,其外层表面与不同的多肽(构成蛋白质的基本单位)相对应,能大大降低干细胞发育方向的不确定性。这种纯化学的培养基面能对细胞的“信号”路径施加一种控制。“信号”是细胞内分子之间互相通讯的方式,比如告知免疫细胞准备抵抗外来感染,或告知胰腺细胞机体需要更多胰岛素等等。通过控制干细胞内部的分子通讯,研究人员能告知干细胞发育成某种特定的细胞。
为了测试这种纯化学培养基面改变信号的能力,研究人员还用β转化生长因子(β-TGF,能控制细胞从生长到毁灭的多种功能活性)对癌细胞进行了测试,实验结果表明,这种培养基面可以促进伤口愈合。基斯林解释说,β-TGF虽然有助于伤口愈合,但如果它接触到健康皮肤,会造成皮肤发炎。而通过这种培养基面,比如设计一种绷带,只在身体局部集中某种特殊的多肽,可让β-TGF只集中在伤口部位。
基斯林认为,要通过组织工程培养出替代器官,人们必须能操控细胞的发展方向。设计多套与各种多肽对应的培养表面,就能引导胚胎干细胞按照需要进行分化。这种培养表面让实验室制造器官和组织更加容易,是一种更先进的干细胞生产方法。
生物探索推荐英文文章阅读:
Controlling cells' environments: A step toward building much-needed tissues and organs
With stem cells so fickle and indecisive that they make Shakespeare’s Hamlet pale by comparison, scientists today described an advance in encouraging stem cells to make decisions about their fate. The technology for doing so, reported here at the 242nd National Meeting & Exposition of the American Chemical Society (ACS), is an advance toward using stem cells in “regenerative medicine” -- to grow from scratch organs for transplants and tissues for treating diseases.
“Stem cells have great potential in regenerative medicine, in developing new drugs and in advancing biomedical research,” said Laura L. Kiessling, Ph.D., who presented the report. “To exploit that potential, we need two things: first, reproducible methods to grow human stem cells in the laboratory, and second, the ability to make stem cells grow into heart cells, brain cells or whatever kind of cell. Our technology takes a different approach to both of these problems, and the results are very encouraging.”
Biologically, so-called pluripotent human embryonic stem cells have not made up their minds about what to become. That’s essential because these cells, which are derived from embryos, have the agility to develop into the hundreds of different kinds of cells in a fully-formed human body. But controlling their differentiation has also stood as a major barrier to making the stem cell dream come true and using these all-purpose cells in medicine.
Past approaches to growing and scripting the fate of stem cells have involved adding growth-regulating and other substances to cultures of stem cells growing in the laboratory. These conditions left scientists guessing about exactly what wound up in the stem cells. Kiessling and colleagues are pioneering a new approach that involves using chemically controlled surfaces.
Kiessling previously developed chemically modified plastic and glass surfaces that take much of the guess work out of growing stem cells in laboratory cultures. In the past, scientists grew stem cells on surfaces that contained mouse cells. That left scientists with nagging questions about possible contamination of stem cells with disease-causing animal viruses — a stumbling block for using stem cells in potential medical applications. And that growth system was what scientists term “undefined.” There were variations from batch to batch of mouse cells, and scientists never really knew what the stem cells were coming into contact with and how it might be changing them. The synthetic, chemically-defined, surfaces ended that uncertainty. The approach was inexpensive, simple and a much-needed advance in producing stem cells, Kiessling explained.
With the ability to grow stem cells on the synthetic surfaces under chemically defined, or known, conditions, Kiessling’s group took an additional step in their latest research. It found that chemically defined surfaces can exert control over signaling pathways. “Signaling” is how molecules talk to one another and get things done inside a cell. It’s how an immune cell knows to fight an infection or how a pancreatic cell determines that more insulin is needed in the bloodstream, for example. By controlling how molecules inside a stem cell communicate, researchers could someday in the future nudge them to become one type of cell or tissue over another.
To see whether a new chemically defined surface could change signaling in a pilot experiment, Kiessling tested cancer cells. The research involved use of a signaling substance, transforming growth factor-beta (TGF-beta), which controls a range of activities, from cell growth to self-destruction.
“The new surfaces give scientists much more control over cells, opening up a wide range of possible future applications,” Kiessling explained. Building directly on the results of the pilot study, the surfaces could have applications in wound healing. TGF- beta can help wounds heal, but if it touches healthy skin, inflammation or even a cancerous tumor could develop. “We haven’t done this, but you could imagine a bandage that has a localized concentration of the special peptide surface that would recruit TGF-beta just to the wound site,” said Kiessling.
The surfaces also could make it easier to manufacture organs and tissues in the laboratory someday. “We think that this strategy, with different sets of peptides (building blocks of proteins) bound to the surface, could direct certain human embryonic stem cells on the surface to become one type of cell and other stem cells to become a second cell type, right next to each other. For the tissue engineering involved in growing replacement organs, you need to organize specialized cells in particular ways like this.”







