据“中央社”报道,美国俄勒冈健康科学大学(OHSU)近日宣布,在猴子身上进行的疫苗实验成功,可能有助于研发用于人类身上的艾滋病毒(HIV)疫苗。
这项医学研究是俄勒冈健康科学大学的疫苗暨基因治疗研究中心(Vaccine and Gene Therapy Institute)、国际艾滋病疫苗行动组织(International AIDSVaccine Initiative,简称IAVI)以及位于马里兰州弗瑞德里克(Frederick)的国家癌症研究所(NationalCancer Institute)联合进行的。
根据研究人员发表在科学杂志《自然》(Nature)网络版的医学报告,研究人员将使用猿猴免疫缺乏病毒(Simian immunodeficiency virus,简称SIV)制成的疫苗,施打在俄勒冈国家灵长类动物研究中心内的恒河猴(rhesus macaque monkeys)身上,结果发现有接受疫苗施打的猴子当中,有半数在经过敏感测试时,已经测不到猿猴免疫缺乏病毒了。
研究人员指出,下一步将是对人类进行临床实验,这项研究结果可能有助于研发用在人类身上的艾滋病毒疫苗。
生物探索推荐英文论文摘要:
Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine
The acquired immunodeficiency syndrome (AIDS)-causing lentiviruses human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) effectively evade host immunity and, once established, infections with these viruses are only rarely controlled by immunological mechanisms. However, the initial establishment of infection in the first few days after mucosal exposure, before viral dissemination and massive replication, may be more vulnerable to immune control. Here we report that SIV vaccines that include rhesus cytomegalovirus (RhCMV) vectors establish indefinitely persistent, high-frequency, SIV-specific effector memory T-cell (TEM) responses at potential sites of SIV replication in rhesus macaques and stringently control highly pathogenic SIVMAC239 infection early after mucosal challenge. Thirteen of twenty-four rhesus macaques receiving either RhCMV vectors alone or RhCMV vectors followed by adenovirus 5 (Ad5) vectors (versus 0 of 9 DNA/Ad5-vaccinated rhesus macaques) manifested early complete control of SIV (undetectable plasma virus), and in twelve of these thirteen animals we observed long-term (≥1 year) protection. This was characterized by: occasional blips of plasma viraemia that ultimately waned; predominantly undetectable cell-associated viral load in blood and lymph node mononuclear cells; no depletion of effector-site CD4+ memory T cells; no induction or boosting of SIV Env-specific antibodies; and induction and then loss of T-cell responses to an SIV protein (Vif) not included in the RhCMV vectors. Protection correlated with the magnitude of the peak SIV-specific CD8+ T-cell responses in the vaccine phase, and occurred without anamnestic T-cell responses. Remarkably, long-term RhCMV vector-associated SIV control was insensitive to either CD8+ or CD4+ lymphocyte depletion and, at necropsy, cell-associated SIV was only occasionally measurable at the limit of detection with ultrasensitive assays, observations that indicate the possibility of eventual viral clearance. Thus, persistent vectors such as CMV and their associated TEM responses might significantly contribute to an efficacious HIV/AIDS vaccine
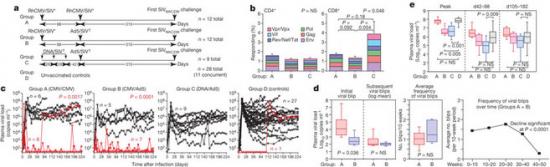
Figure 1: Immunogenicity and efficacy of RhCMV/SIV vectors.







