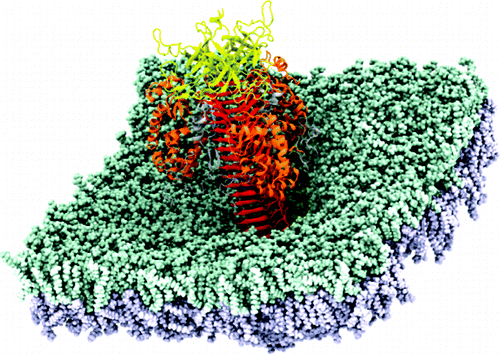
“瑞士军刀”蛋白正穿透细菌表膜(图)
为了深刻的理解大自然的“复制机”、“马达”、“装配线”和其它各种生物学上的毫微级机器,日本科学家最新的研究向我们描述了一种病毒尾部像“瑞士军刀”的多功能蛋白是如何像一个钻头钻入细菌表膜的。
这项研究的论文发表在最新一期的《Journal of the American Chemical Society》杂志上。
论文的通讯作者,日本的Akio Kitao教授将研究锁定在了名为“噬菌体”的一系列病毒中。这些病毒能感染细菌并使其分解,如大肠杆菌。它们会向细菌中注入它们的DNA或RNA,使其控制整个细菌。而完成该步骤需要使用许多不可见的毫微级工具。
科学家们再现了病毒感染大肠杆菌尾部蛋白的详细过程。电脑模型显示蛋白质有规律的发挥着功能:该蛋白开始像一个螺钉一样刺入大肠杆菌的外部膜,然后像一个细胞穿孔机一样将膜上的残骸移开,并一步步的将穿孔放大,直至穿透到细胞膜内,并最终将遗传物质注入细菌。这项研究展示了一个单一功能的蛋白质如何通过其结构的各个部分完成一项复杂的侵染过程的。(生物探索 Jun译)
生物探索推荐英文论文原文摘要:
Screw Motion Regulates Multiple Functions of T4 Phage Protein Gene Product 5 during Cell Puncturing
Bacteriophage T4 penetrates the outer membrane of Escherichia coli using a multifunctional device composed of a gene product 5 (gp5) protein trimer. We report that gp5 sequentially exerts distinct functions along the course of penetration stages induced by screw motion. A triple-stranded β-helix of gp5 acts as a cell-puncturing drill bit to make a hole on the membrane and then send the lipids upward efficiently by strong charge interactions. The gp5 lysozyme domains, which degrade the peptidoglycan layer later, are shown to play novel roles to enlarge the hole and control the release of the β-helix. The lysozyme active site is protected from lipid binding during the penetration and is exposed after the β-helix release. Intrinsic multiple functions of gp5 are shown to be served in turn regulated by gradual change of interdomain interactions, which enables the initial infection process with single protein trimer by continuous screw motion. The results of lysozyme domain should be understood as the case where a single-function protein acquired multiple chemical functions through interplay with other domains in a multidomain protein.







