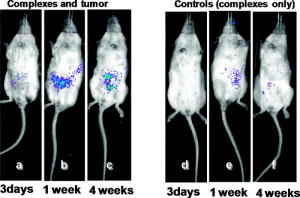
荧光照相结果显示该丝蛋白能靶向结合在癌细胞上(图)
在最新一期的《Bioconjugate Chemistry》杂志上有一篇文章报道,科学家们用基因工程的手段研发了一种蜘蛛丝用作基因治疗的“载体”,目前在实验室动物身上取得了成功。
美国马萨诸塞州塔夫茨大学David Kaplan的研究小组发现基因治疗手段(使用有利于人体的基因来预防或治疗疾病)需要高效、安全的运输方式或“载体”。这些“载体”应该能构成药片和胶囊的一部分,并能有效的将这些治疗用的基因转入人体的细胞中。目前试验中广泛使用病毒作为载体,由于这种基因转导方式使得各种基因治疗始终无法通过FDA的认证,尽管从1989年至今已经几乎有1500例临床治疗病例。现在,它们发现了一种新的基因运输“载体”—丝蛋白,它将非常适用于未来生物药物和生物治疗手段的开发。
这种经过基因工程改造够的蜘蛛丝蛋白能有效的粘附发生病变的细胞,而不是正常的细胞。他们还在丝蛋白中加入了能编码荧光蛋白的基因,以便于观察基因是否运输到了指定的地方(使用特定的设备)。科学家们用带有人类乳腺癌细胞的小鼠进行了实验,发现丝蛋白能有效的粘附在这些癌细胞上,并将质粒DNA物质以无害的方式转入细胞中。
在未来的基因治疗中,这项研究发现会是取代病毒载体进行基因转导的更好的“平台”。
生物探索推荐英文论文原文摘要:
Spider Silk-Based Gene Carriers for Tumor Cell-Specific Delivery
The present study demonstrates pDNA complexes of recombinant silk proteins containing poly(l-lysine) and tumor-homing peptides (THPs), which are globular and approximately 150–250 nm in diameter, show significant enhancement of target specificity to tumor cells by additions of F3 and CGKRK THPs. We report herein the preparation and study of novel nanoscale silk-based ionic complexes containing pDNA able to home specifically to tumor cells. Particular focus was on how the THP, F3 (KDEPQRRSARLSAKPAPPKPEPKPKKAPAKK), and CGKRK, enhanced transfection specificity to tumor cells. Genetically engineered silk proteins containing both poly(l-lysine) domains to interact with pDNA and the THP to bind to specific tumor cells for target-specific pDNA delivery were prepared using Escherichia coli, followed by in vitro and in vivo transfection experiments into MDA-MB-435 melanoma cells and highly metastatic human breast tumor MDA-MB-231 cells. Non-tumorigenic MCF-10A breast epithelial cells were used as a control cell line for in vitro tumor-specific delivery studies. These results demonstrate that combination of the bioengineered silk delivery systems and THP can serve as a versatile and useful new platform for nonviral gene delivery.







