摘要:过去的十年间,研究人员试图重新编码各种细胞类型。研究人员希望通过新组织的移植进而修复受损的心肌,因此,心肌细胞是再生医疗中最紧缺的细胞之一。现在,宾夕法尼亚大学佩雷尔曼医学院的研究人员首次人为地实现由非心肌细胞直接转化成心肌细胞。
基于这一想法——细胞的“标签”取决于mRNA(后者在指导蛋白质合成过程中提供了细胞分化蓝图),研究人员通过mRNA转移技术把两种细胞类型——星形胶质细胞(星形脑细胞)和成纤维细胞(皮肤细胞)——转分化成心肌细胞,该研究使得心血管疾病的细胞疗法成为可能。
埃尔默霍姆斯博斯特(Elmer Holmes Bobst)药理学教授James Eberwine 博士、Tae Kyung Kim博士及同事把相关的研究发表在网络版的Proceedings of the National Academy of Sciences期刊上。
Eberwine博士称,心肌细胞再生技术的新颖之处是通过RNA转移技术将一种细胞类型转分化成另外一种细胞,没有去分化的中间环节。科学家利用脂质体介导的转染技术把心肌细胞的mRNAs递送到星性胶质细胞或成纤维细胞,受体细胞可行驶其它生理功能。外源的RNA转录谱改变受体细胞核DNA的转录模式,最终实现向目标细胞的转化。
研究小组使用的技术称为转录谱诱导表型重塑(Transcriptome Induced Phenotype Remodeling)或TIPeR。此技术与诱导多能干细胞技术(iPS)区别之处是不需要脱分化成多能干细胞和在生长因子用下再分化成目标细胞。之前,类似的核转化技术把一种细胞核转化成另外一种细胞核;基于改变的RNA转录谱,转化的细胞核介导细胞表型的转变。
研究小组首次从心肌细胞中抽提出mRNA,再注射到受体细胞中。与星形胶质细胞或成纤维细胞相比,心肌细胞具有更多的mRNAs,其中包括组织特异性mRNAs。心肌细胞的mRNAs在受体细胞质中表达成蛋白质,进而影响受体细胞核的基因表达,最终实现受体细胞向心肌细胞的转化以及心肌细胞特异蛋白的高丰度表达。
为了追踪星形细胞向心肌细胞转化,研究小组通过单细胞微阵列分析、细胞形状和免疫电学特性观测新细胞RNA转录谱。TIPeR技术可潜在用于心肌细胞治疗,并在药物治疗方面筛选出个体化用药以及用于开发新药。
多学科的研究人员参与这一研究,包括心血管内科 Vickas Patel博士 和 Nataliya Peternko以及生物系Miler Lee博士和 Junhyong Kim博士等。此外,多个机构对这一研究提供资助。(生物探索译 Pobee)
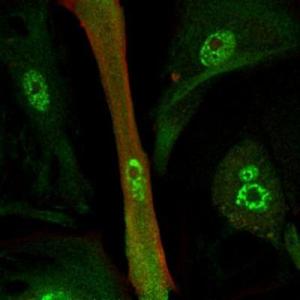
重编码的心肌细胞 (绿色和红色表示心肌蛋白分布)
生物探索推荐英文原文
A Change of Heart: Researchers Reprogram Brain Cells to Become Heart Cells
For the past decade, researchers have tried to reprogram the identity of all kinds of cell types. Heart cells are one of the most sought-after cells in regenerative medicine because researchers anticipate that they may help to repair injured hearts by replacing lost tissue. Now, researchers at the Perelman School of Medicine at the University of Pennsylvania are the first to demonstrate the direct conversion of a non-heart cell type into a heart cell by RNA transfer.
Working on the idea that the signature of a cell is defined by molecules called messenger RNAs (mRNAs), which contain the chemical blueprint for how to make a protein, the investigators changed two different cell types, an astrocyte (a star-shaped brain cell) and a fibroblast (a skin cell), into a heart cell, using mRNAs.
James Eberwine, PhD, the Elmer Holmes Bobst Professor of Pharmacology, Tae Kyung Kim, PhD, post-doctoral fellow, and colleagues report their findings online in the Proceedings of the National Academy of Sciences. This approach offers the possibility for cell-based therapy for cardiovascular diseases.
"What's new about this approach for heart-cell generation is that we directly converted one cell type to another using RNA, without an intermediate step," explains Eberwine. The scientists put an excess of heart cell mRNAs into either astrocytes or fibroblasts using lipid-mediated transfection, and the host cell does the rest. These RNA populations (through translation or by modulation of the expression of other RNAs) direct DNA in the host nucleus to change the cell's RNA populations to that of the destination cell type (heart cell, or tCardiomyocyte), which in turn changes the phenotype of the host cell into the destination cell.
The method the group used, called Transcriptome Induced Phenotype Remodeling, or TIPeR, is distinct from the induced pluripotent stem cell (iPS) approach used by many labs in that host cells do not have to be dedifferentiated to a pluripotent state and then redifferentiated with growth factors to the destination cell type. TIPeR is more similar to prior nuclear transfer work in which the nucleus of one cell is transferred into another cell where upon the transferred nucleus then directs the cell to change its phenotype based upon the RNAs that are made. The tCardiomyocyte work follows directly from earlier work from the Eberwine lab, where neurons were converted into tAstrocytes using the TIPeR process.
The team first extracted mRNA from a heart cell, then put it into host cells. Because there are now so many more heart-cell mRNAs versus astrocyte or fibroblast mRNAs, they take over the indigenous RNA population. The heart-cell mRNAs are translated into heart-cell proteins in the cell cytoplasm. These heart-cell proteins then influence gene expression in the host nucleus so that heart-cell genes are turned on and heart-cell-enriched proteins are made.
To track the change from an astrocyte to heart cell, the team looked at the new cells' RNA profile using single cell microarray analysis; cell shape; and immunological and electrical properties. While TIPeR-generated tCardiomyocytes are of significant use in fundamental science it is easy to envision their potential use to screen for heart cell therapeutics, say the study authors. What's more, creation of tCardiomyoctes from patients would permit personalized screening for efficacy of drug treatments; screening of new drugs; and potentially as a cellular therapeutic.
These studies were enabled through the collaboration of a number of investigators spanning multiple disciplines including Vickas Patel, MD and Nataliya Peternko from the Division of Cardiovascular Medicine, Miler Lee, PhD and Junhyong Kim, PhD from the Department of Biology and Jai-Yoon Sul, PhD and Jae Hee Lee, PhD also from the Department of Pharmacology, all from Penn. This work was funded by grants from the W. M. Keck Foundation, the National Institutes of Health Director's Office, and the Commonwealth of Pennsylvania.







