我国已经进入老龄化社会,神经退行性疾病给个人、家庭、社会造成了沉重的经济和精神负担。神经元的变性死亡是神经退行性疾病的重要病理机制。
中国科学院生物物理研究所脑与认知科学国家重点实验室赫荣乔研究组在蛋白质的硝基化修饰方面取得了进展,并于7月6日在J Mol Cell Biol上发表了该项研究成果。刘延英博士后在站期间,发现硝基化可以导致a-synuclein错误折叠,形成具有神经细胞毒性的聚集物。
该成果阐释了硝基化a-synuclein错误折叠聚积产物引起细胞死亡的分子机制,为突触核蛋白相关神经退行性疾病的研究提供了新的思路。
生物探索推荐英文原文:
A novel molecular mechanism for nitrated a-synuclein-induced cell death
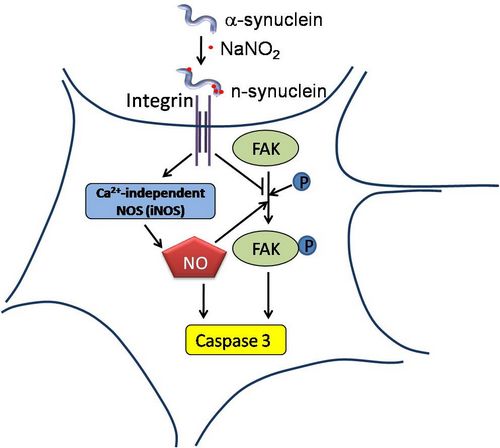
Abstract:Although previous studies have demonstrated the involvement of nitrated a-synuclein in neurodegenerative disorders (synucleinopathies), the effects of nitrated a-synuclein and the molecular mechanisms underlying its toxicity are still unclear. In the presentstudy, nitrated a-synuclein with four 3-nitrotyrosines was obtained non-enzymatically by incubationwith nitrite. The nitrated protein existed as a mixture of monomers, dimers, and polymers in solution. The nitrated a-synuclein couldinduce cell death in a time- and concentration-dependent manner when SH-SY5Y cells (a human neuroblastoma cell line) were incubated with the dimers and polymers. Treatment with anti-integrin a5b1 antibody partially rescued the SH-SY5Y cells from the celldeath. Dot blotting and immunoprecipitation revealed that the nitrated protein bound to integrin on the cell membranes. Level ofnitric oxide (NO) and calcium-independent inducible NO synthase (iNOS) activity increased during the initial stages of the treatment.
The expression of phosphorylated focal adhesion kinase (FAK) decreased in the cells. Subsequently, an increase in caspase 3activity was observed in SH-SY5Y cells. Our results demonstrate that activation of iNOS and inhibition of FAK may both be responsible for the celldeath induced by nitrated a-synuclein. These data suggest that the cytotoxicity of nitrated a-synuclein is mediatedvia an integrin-iNOS/-FAK signaling pathway, and that the nitration of a-synuclein plays a role in neuronal degeneration.







