加州大学圣地亚哥分校的研究者开发出了将纳米微粒伪装成红细胞的新方法,使之绕过人体免疫系统、直接将抗癌药递送到肿瘤部位。本研究将于下周发表于《美国国家科学院院刊》。
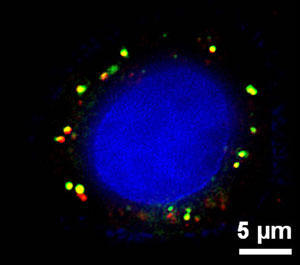
荧光显微镜下的纳米微粒药物
扫描荧光显微图像显示红细胞膜被覆的聚合纳米粒被一个肿瘤细胞接受后的情形。绿色染料显示红细胞膜,红色染料显示聚合体核心,而兰色染料则显示癌细胞。(照片来源:美国国家科学院院刊)
本方法涉及利用来自红血球的细胞膜,以此包裹可生物降解的多聚体纳米微粒(内含小分子抗癌药),使之拥有强大的伪装以避免免疫系统攻击。纳米微粒的尺寸小于100纳米,跟病毒的个头差不多。
美国圣地亚哥雅各斯工程学院与莫雷斯UCSD中心的纳米工程学教授Liangfang Zhang 说:“这是科学家首次将天然的细胞膜与纳米微粒相结合并应用于药物递送。该纳米微粒平台基本上不存在激发免疫响应的风险。”
多年来,研究者一直在开发模仿人体天然行为的药物递送系统,以求更有效地递送药物。这意味着:必须创建诸如纳米微粒之类能够在体内持续存活并循环、同时又免受免疫系统攻击的运载工具。 红细胞在体内的存活时间为180天左右,同时,正如Zhang的UCSD生物工程博士生、本论文第一作者Che-Ming Hu所形容的,红细胞也是“天然的、长时间循环的递送工具。”
隐形纳米微粒目前已成功应用于临床癌症治疗,以此递送化疗药物。其包膜的合成材料是聚乙二醇之类,形成一个保护层以抑制免疫系统,从而为纳米粒递送有效载荷争取时间。Zhang说,目前的隐形纳米粒药物递送工具能够在体内循环数个小时,比不采用特殊包膜时只存在几分钟时间来说还算可以。
但在本研究中,用红细胞膜包裹的纳米粒在体内循环时间长达两天左右。研究资金来自美国国家卫生研究院。
一项向个体化医疗的转变
应用人体本身的红细胞既标志着研究焦点的重大转移,也标志着个体化药物递送研究领域的一项重大突破。要想在合成包膜上模仿红细胞的首要属性,需要深入理解所有蛋白与脂质在细胞表面运作的具体过程,只有这样才能使包膜模仿出正确的属性。但Zhang的研究小组则快刀斩乱麻,干脆直接利用了真正红细胞的完整的细胞膜。
Zhang对此作了解释:“我们是从工程学角度着手解决这一问题的,因此绕过了生物学理解上的瓶颈。如果红细胞有我们需要的特征,并且,我们也知道该特征与膜有关,那么,虽然我们并未完全搞通蛋白水平上的相关原理,直接利用完整的细胞膜可能不失为良策。把膜包在纳米微粒上,让微粒象红细胞,说不定就行。”
通常情形下,通过静脉缓慢滴注化疗药液要几个小时,而利用纳米微粒来递送药物(单次注射)则将时间减少到几分钟。这样病人的感受大为改善,也使治疗计划得以顺利展开。本突破使个体化药物递送水平更佳,只要小样本的病人本身的血液即可提供足够多的红细胞膜来伪装纳米微粒,并且几乎将免疫反应降为零。
Zhang说,接下来的步骤之一是开发大规模生产这些仿生纳米微粒以供临床使用的方法。 美国国家科学基金会将此提供资金支持。研究者也将在膜上添加靶分子,使微粒能够(自动)寻找肿瘤细胞并与之结合;同时,该研究小组的药物加载技术将使纳米微粒内核多功能化,使之能够同时递送多种药物。
Zhang说,一颗纳米粒能够递送多种药物很重要,因为肿瘤细胞会对单一药物产生抗性。如此多管齐下,纳米微粒就能瞄准癌细胞,所产生的“火力”会象炸弹一样在癌细胞内部爆炸。
生物探索推荐英文原文
DOI: 10.1073/pnas.1106634108
Nanoparticles Disguised as Red Blood Cells to Deliver Cancer-Fighting Drugs
Researchers at the University of California, San Diego have developed a novel method of disguising nanoparticles as red blood cells, which will enable them to evade the body's immune system and deliver cancer-fighting drugs straight to a tumor. Their research will be published next week in the online Early Edition of the Proceedings of the National Academy of Sciences.
The method involves collecting the membrane from a red blood cell and wrapping it like a powerful camouflaging cloak around a biodegradable polymer nanoparticle stuffed with a cocktail of small molecule drugs. Nanoparticles are less than 100 nanometers in size, about the same size as a virus.
"This is the first work that combines the natural cell membrane with a synthetic nanoparticle for drug delivery applications." said Liangfang Zhang, a nanoeningeering professor at the UC San Diego Jacobs School of Engineering and Moores UCSD Cancer Center. "This nanoparticle platform will have little risk of immune response."
Researchers have been working for years on developing drug delivery systems that mimic the body's natural behavior for more effective drug delivery. That means creating vehicles such as nanoparticles that can live and circulate in the body for extended periods without being attacked by the immune system. Red blood cells live in the body for up to 180 days and, as such, are "nature's long-circulation delivery vehicle," said Zhang's student Che-Ming Hu, a UCSD Ph.D. candidate in bioengineering, and first author on the paper.
Stealth nanoparticles are already used successfully in clinical cancer treatment to deliver chemotherapy drugs. They are coated in a synthetic material such as polyethylene glycol that creates a protection layer to suppress the immune system so that the nanoparticle has time to deliver its payload. Zhang said today's stealth nanoparticle drug delivery vehicles can circulate in the body for hours compared to the minutes a nanoparticle might survive without this special coating.
But in Zhang's study, nanoparticles coated in the membranes of red blood cells circulated in the bodies of lab mice for nearly two days. The study was funded through a grant from the National Institute of Health.
A shift towards personalized medicine
Using the body's own red blood cells marks a significant shift in focus and a major breakthrough in the field of personalized drug delivery research. Trying to mimic the most important properties of a red blood cell in a synthetic coating requires an in-depth biological understanding of how all the proteins and lipids function on the surface of a cell so that you know you are mimicking the right properties. Instead, Zhang's team is just taking the whole surface membrane from an actual red blood cell.
"We approached this problem from an engineering point of view and bypassed all of this fundamental biology," said Zhang. "If the red blood cell has such a feature and we know that it has something to do with the membrane -- although we don't fully understand exactly what is going on at the protein level -- we just take the whole membrane. You put the cloak on the nanoparticle, and the nanoparticle looks like a red blood cell."
Using nanoparticles to deliver drugs also reduces the hours it takes to slowly drip chemotherapy drug solutions through an intravenous line to just a few minutes for a single injection of nanoparticle drugs. This significantly improves the patient's experience and compliance with the therapeutic plan. The breakthrough could lead to more personalized drug delivery wherein a small sample of a patient's own blood could produce enough of the essential membrane to disguise the nanoparticle, reducing the risk of immune response to almost nothing.
Zhang said one of the next steps is to develop an approach for large-scale manufacturing of these biomimetic nanoparticles for clinical use, which will be done through funding from the National Science Foundation. Researchers will also add a targeting molecule to the membrane that will enable the particle to seek and bind to cancer cells, and integrate the team's technology for loading drugs into the nanoparticle core so that multiple drugs can be delivered at the same time.
Zhang said being able to deliver multiple drugs in a single nanoparticle is important because cancer cells can develop a resistance to drugs delivered individually. By combining them, and giving the nanoparticle the ability to target cancer cells, the whole cocktail can be dropped like a bomb from within the cancer cell.







