瑞典卡罗林斯卡研究所(Karolinska Institutet)的科学家发现,诵读障碍症基因具有令人吃惊的生理功能:它能调控天线状纤毛的生理结构——细胞可借此进行交流。
诵读障碍症很大程度上具有遗传性,并与许多基因相关,然而,这类基因的大多数功能却知之甚少。DCDC2属于这类基因,参与调控神经元上纤毛的信号传导,瑞典卡罗林斯卡医学院和芬兰赫尔辛基大学研究发现这一基因功能。
赫尔辛基大学Eero Castrén教授和Juha Kere教授共同领导这一研究,后者称,我们的研究可能提供在神经生物学方面诵读障碍症的病理。
纤毛是大多数细胞表面隆起的毛发状结构,长期以来,它们的功能一直是个谜,但最近的研究表明,纤毛可供细胞交流使用,并在机体器官发育过程中发挥关键作用。
这一研究结论和之前小鼠的研究共同支持,DCDC2基因和其它2种诵读障碍症相关基因一起参与细胞迁移的调控,在胚胎发育过程中,神经细胞可在大脑中准确定位到相应区域。
Kere教授称,纤毛是“动力装置”——控制细胞迁移——的重要组成“部件”,我们感兴趣的问题是,这些纤毛的生理功能是什么?以及诵读障碍症是怎样形成的?这一问题的解答还需要进一步的研究。
DCDC2蛋白可调控纤毛的长度,并能参考细胞的活跃状态激活2种不同的信号系统。人体中该基因变异体植入蛔线虫神经细胞中,不同寻常的神经信号传递仅仅发生在含有纤毛的神经细胞中。这些新的研究结论发表在PLoS ONE科学杂志上。
不同器官的纤毛功能障碍与大量的疾病有关,这些疾病包括罕见的遗传性疾病(如多囊肾病和Kartagener综合征)和其它类疾病,如糖尿病、肥胖和精神分裂症等。(生物探索译文)
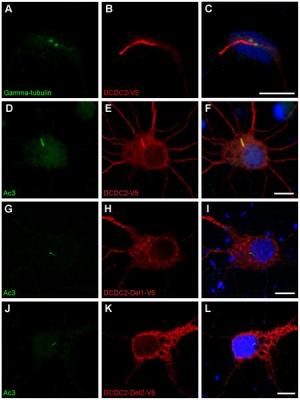
DCDC2蛋白定位在神经细胞的纤毛上
生物探索推荐英文摘要
Unexpected Function of Dyslexia-Linked Gene: Controlling Cilia of Cells
Abstract: Scientists at Karolinska Institutet in Sweden have discovered that a gene linked to dyslexia has a surprising biological function: it controls cilia, the antenna-like projections that cells use to communicate.
Dyslexia is largely hereditary and linked to a number of genes, the functions of which are, however, largely unknown. This present study from Karolinska Institutet and Helsinki University now shows that one of these genes, DCDC2, is involved in regulating the signalling of cilia in brain neurons.
"Our discovery presents us with a possible new neurobiological mechanism for dyslexia," says Professor Juha Kere, who co-led the study with Professor Eero Castrén of Helsinki University.
Cilia are hair-like structures that project from the surface of most cells. Their purpose has long remained something of a mystery, but recent research has revealed that the cells use them to communicate and that they play a crucial part in the development of the body's organs.
These results tie into previous research in mice showing that DCDC2 and two other dyslexia genes are involved in cell migration, a process by which nerve cells are moved to their correct location in the brain during embryonic development.
"The cilia are important parts of the machinery that controls cell migration," says Professor Kere. "Just what they do and how it could result in dyslexia are interesting questions that will be given further study."
The new findings, which are presented in the scientific journal PLoS ONE show that DCDC2 governs the length of the cilia and activates two different signal systems in the cell, depending on the degree of gene activity. When the human variant of the gene was transferred to nerve cells in the roundworm C. elegans, it gave rise to unusual neural projections exclusively in ciliated cells.
Ciliary dysfunction in different organs has been associated with a wide range of disorders from rare genetic diseases such as polycystic kidney disease and Kartagener's syndrome, to diabetes, obesity and schizophrenia.







