目前欧洲大肠杆菌疫情肆虐,女人、豆芽、黄瓜、细菌、牛,这些都是当下欧洲大肠杆菌爆发大戏的演员阵容,现在,我们应该把噬菌体也加进去了,噬菌体是一种可以感染细菌的病毒,它是引起这次疫情的诸多因素中非常关键的一环,当大肠杆菌感染了可以产生志贺毒素的噬菌体时,就能威胁到人类健康。
细菌感染往往由被污染的食物引起,但是现在疫情已经爆发六周之久了,如何发生的线索正在逐渐消失。确定究竟谁是真凶已经非常困难,但是通过此事,必须对传染性细菌如何进入食物链这一问题引起足够的重视。
德国疫情中病人的案例研究将矛头指向了沙拉蔬菜,黄瓜和豆芽也都存在嫌疑。这些蔬菜很可能来自土壤和水源中的细菌污染,但更有可能的是动物带来了这些细菌。致病性的大肠杆菌通常由反刍动物(牛羊)传染给人类,传播途径包括:通过其粪便污染食物链,或者通过生牛奶和肉类的消费来传播。
但是,致病性大肠杆菌如何变得如此可怕?这便是噬菌体干的好事了。本次爆发的细菌被认为是 O104:H4,它可以产生志贺毒素。这种毒素通常会导致病人严重腹泻和肾脏损伤,而大肠杆菌会导致进一步恶化为溶血性尿毒症。志贺毒素的基因实际上并不是细菌的基因,而噬菌体的基因表现为传染性细菌。所以,当大肠杆菌被产生志贺毒素的噬菌体感染后,就变得具有致病性了。
我们使用的抗生素可能会帮助那些病毒性基因传播。当一个细菌遭遇到某种它曾经经历过的一种抗生素时(称为SOS反应),噬菌体的复制也就开始了。大量复制的噬菌体将导致细菌细胞迸裂,释放出噬菌体。与此同时,毒素也会被释放,这就是为什么抗生素往往对治疗大肠杆菌感染无效。
保护的代价
O104:H4 菌株诸多不寻常的特征之一是它具有对多种抗生素的耐药性。这意味着不论细菌从何而来,都会对一些抗生素具备一定的抵抗能力。英国利物浦大学的微生物学家 Heather Allison(希瑟-艾莉森)和位于华盛顿特区的食品安全咨询公司的执行董事 David Acheson(大卫-艾奇逊)认为:在农业和环境中滥用抗生素可能会促进能够生成志贺毒素的噬菌体的传播。
Acheson 在美国马萨诸塞州德福德的塔夫特大学领导一个研究小组的时候曾对此课题展开过研究,上世纪九十年代他们曾从分子层面研究了志贺毒素引起的大肠杆菌的致病机理。Acheson 表示,他的团队在对老鼠的实验中曾经观察到,产生志贺毒素的噬菌体在面对丙环沙星这种治疗用的抗生素时,可以在体外和肠道内游走,对此Acheson 非常肯定。但是同时他也指出,这只是实验室观察的结果。
农业中使用的抗生素可能是罪魁祸首。“反刍动物胃里的噬菌体特别多。”设于意大利罗马的欧洲参考实验室的 Alfredo Caprioli(阿尔弗雷多-卡普里奥里)说道。而且,胃部只是噬菌体在不同细菌中穿梭以及新致病菌株产生的一个地方。
由志贺毒素引起的腹泻已经困扰人类数个世纪了,这一类细菌被称为志贺氏杆菌。在1897年日本爆发痢疾传染病时,日本医学博士樱冢澈志贺发现了这种细菌并第一个将其命名为志贺毒素。根据 Allison 的说法,能生成志贺毒素的噬菌体可能继承了志贺毒素的基因编码,1980年代以后,这些剧毒的基因被传播到了其他细菌中,这其中就包括许多大肠杆菌菌株。
产生志贺毒素的噬菌体究竟如何在短短几十年里有如此广泛的传播?Allison 认为这是由于他们与众不同的特性。他们通过附着在一种叫做 BamA 的蛋白质的表面来感染细菌,这将使他们可以有更广阔的寄宿区域。大多数噬菌体只能对一个宿主细胞进行一次感染,但是志贺毒素生成的噬菌体可以多次感染同一个细胞,这将是它们致病的潜在可能性大大提升。而且他们还可以在宿主之外生存,比如土壤和水源中。
Weiss 补充说明道,那些被噬菌体寄宿的细菌同时获得了生存优势,他解释道:一旦这些细菌暴露在环境当中,比如在肥料中,它们就将吞噬微生物生存,比如原生动物。这些毒素可以杀死其他的微生物,让这些细菌更有生存优势。
并非只有大肠杆菌菌株被志贺毒素感染,似乎其他不同种类的细菌也同样被感染。 O104:H4菌株的基因组已经被识别,它与大肠杆菌菌株的基因有很大相似之处。
能产生志贺毒素的噬菌体的不断增加意味着更加不寻常而危险的菌群很可能即将出现。
生物探索推荐英文原文:
Phage on the rampage
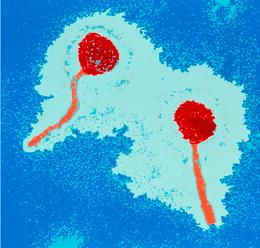
Pictured :Escherichia coli bacteria become dangerous to humans when they are infected by Shiga-toxin-producing bacteriophages.
Women, beansprouts, cucumbers, bacteria, cows: the cast of the current European Escherichia colioutbreak is already a crowd. Enter the phage. Bacteriophages are viruses that infect bacteria, and they are star players in the chain of events that led to this outbreak.
Bacterial infections often originate from contaminated food, but it is now about six weeks since the start of this outbreak and the trail is going cold. It's hard to be sure of the culprit — but this simply serves to highlight the importance of understanding how infectious bacteria get into the food chain in the first place.
Case-control studies of patients in the German outbreak pointed to salad vegetables, and both cucumbers and beansprouts have been suspects. It is possible that the vegetables were contaminated with bacteria originally carried in soil or water; but the more likely source of the bacteria is animals. Pathogenic E. coli are typically passed to humans from ruminant animals (cows or sheep) via faecal contamination in the food chain or through consumption of raw milk or meat products.
But how do pathogenic E. coli arise in the first place? This is where bacteriophage come in. The bacterium in this outbreak, currently recognised as strain O104:H4, makes Shiga toxin, which is responsible for the severe diarrhoea and kidney damage in patients whose E. coliinfections develop into haemolytic uremic syndrome (HUS). The genes for the Shiga toxin are not actually bacterial genes, but phage genes being expressed by infected bacteria. So when an E. coli bacterium gets infected with a Shiga-toxin-producing phage, it becomes pathogenic to humans.
Our use of antibiotics may be helping those viral genes to spread. If bacteria are exposed to some types of antibiotics they undergo what is called the SOS response, which induces the phage to start replicating. Active replication of the phage causes the bacterial cells to burst open, which releases the phage. It also releases the toxin, which is why antibiotics are not usually used to treat E. coliinfections (see 'Europe's E. coli outbreak: time for the antibiotics?').
The cost of protection
One of the many unusual characteristics of strain O104:H4 is that it has resistance genes to multiple classes of antibiotics. This suggests that wherever the bacteria have come from, there has been selective pressure to resist antibiotics. Heather Allison, a microbiologist at the University of Liverpool, UK, and David Acheson, a managing director for food safety at consulting firm Leavitt Partners in Washington DC, agree it is plausible that exposure to antibiotics — in agricultural use or in the environment — might be enhancing the spread of Shiga-toxin-producing phage.
Acheson worked on this question when he led a research group at Tufts University in Medford, Massachusetts, studying the molecular pathogenesis of Shiga-toxin-producing E. coli in the 1990s. He says they saw Shiga-toxin-producing phage transfer between E. coli in response to sub-therapeutic levels of the antibiotic ciprofloxacin in vitro and in the intestines of mice.
"They do it in the laboratory," he says, "but it's hard to show it happens in the environment." He is convinced it does, though. "The potential for the creation of new pathogens via phage release is absolutely a factor in the broader environmental danger of overuse of antibiotics."
Agricultural use of antibiotics is a possible suspect. "Phage are particularly abundant in the guts of ruminants", says Alfredo Caprioli, from the European Reference Laboratory for verotoxin-producing E. coli in Rome, Italy (verotoxin is another name for Shiga toxin). And the gut is one place in which the phage move between different bacteria, and new pathogenic bacterial strains emerge.
Shiga toxins have been causing diarrhoeal disease in humans for centuries — the bacterial genus Shigella and the Shiga toxins were first named for Kiyoshi Shiga, a Japanese medical doctor who identified the bacterium during an outbreak of dysentery in Japan in 1897. According to Allison, Shiga-toxin producing phage probably picked up the genes encoding Shiga toxin from these bacteria, and since the 1980s have been spreading these virulent genes to other bacteria, including many strains of E. coli.
"We are seeing more and more Shiga-toxin-producing strains," says Alison Weiss, microbiologist at the University of Cincinnati in Ohio.
How have Shiga-toxin-producing phage spread so widely in just a few decades? Allison says they have unusual characteristics that make them very successful. They infect bacteria by binding to a protein called BamA on the surface of many bacterial cells, which gives them a broad range of hosts. Most phage can only infect a host cell once, but Shiga-toxin-producing phage can infect the same cell multiple times, giving them greater pathogenic potential. And they can survive outside their hosts, in water or soil, for example.
Weiss adds that carrying the phage also provides a survival advantage for the host bacteria. "Once the bacteria are out in the environment — say in manure — they are fed on by other microbes, such as protozoans. The toxins kill the other microbes, giving these bacteria an advantage."
Not only are more E. coli strains being infected with Shiga toxin, but it seems to be moving into different classes of bacteria. The genome of strain O104:H4 has been sequenced, and it shares many genes with enteroaggerative E. coli (EAEC) strains. "EAEC strains are not typically associated with zoonotic infections, and EAEC and Shiga toxin is a very unusual combination," says Caprioli.
This increased movement of Shiga-toxin-producing phage means that even more unusual and dangerous strains could be on the horizon.
Published online 9 June 2011 | Nature | doi:10.1038/news.2011.360