大多数哺乳动物雄性与雌雄生殖道之间存在一个天然的渗透压差(以小鼠为例:雄性附睾~415 mOsm, 雌性子宫~310 mOsm)。精子从雄性生殖道进入雌性生殖道,经历了一个生理状态下的“低渗应激”。这种应激一方面有利于激活精子运动(从进化上保留了鱼类精子的特征),但低渗环境同时也是一把“双刃剑”,可导致精子细胞过度肿胀对其功能产生潜在伤害。为了消除低渗导致的负面影响,精子在进化中形成了一套高效的液体调节系统以应对这种生理性“低渗应激”导致的细胞肿胀。然而,在此过程中起关键作用的精子蛋白尚未被发现。
动物研究所段恩奎研究组与浙江省医学科学院石其贤教授,吉林大学白求恩第二医院麻彤辉教授合作研究发现:一个水通道蛋白(AQP3)在小鼠和人的精子尾部呈现特异的细胞膜定位。AQP3缺陷的精子在进入雌性生殖道后,虽然能够实现运动激活,但很快表现出大量精子尾部发生弯曲变形。渗透压梯度实验及精子游动中实时录像监测等手段进一步证实,AQP3缺陷精子对低渗导致的细胞膨胀抵御能力下降,在相对低渗的雌性生殖道环境中发生细胞膜进行性膨胀,进而对精子尾部产生机械性牵张并最终导致尾部变形。体内外功能实验表明,由于存在尾部缺陷,大量AQP3敲除鼠精子不能有效地穿越子宫-输卵管结合处狭部,导致精子进入输卵管与卵子相遇的机会降低,从而表现出雄性小鼠生育力下降。
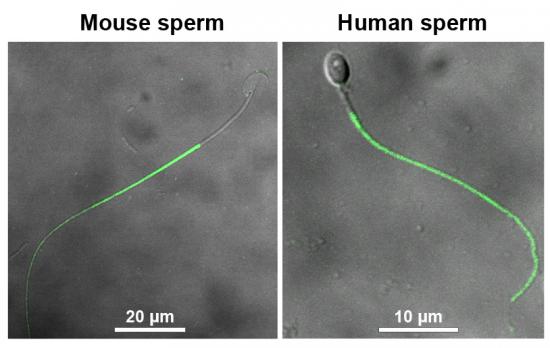
Figure.1 Immunofluorescence staining of AQP3 (green signal) reveals intensive localization at principal piece of mouse and human sperm tail.
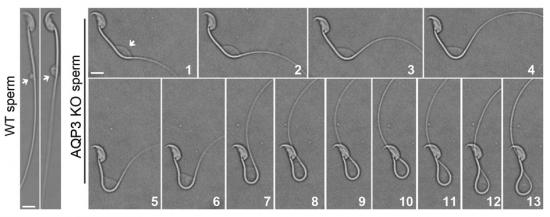
Figure.2 Time-lapse imaging reveals sperm tail bending (Aqp3 null sperm) process forced by progressive membrane expansion upon physiological hypotonic stress. The cell swelling begins at cytoplasmic droplet (indicated by arrows). Note the wildtype sperm only shows mild swelling.
此研究首次揭示了AQP3是精子在雌性生殖道中实现低渗适应的一个关键蛋白,在“低渗应激”介导的精子运动激活和细胞过度肿胀这一对利弊权衡(trade-off)中起到消除负面影响的作用,从而最大优化精子功能。鉴于AQP3在人精子中的表达与小鼠呈现相同模式,其在男性不育/低生育力患者中的作用尚待进一步研究。
本研究于12月7日在线发表于Cell research。本研究得到了国家“发育与生殖”重大科学研究计划和中国科学院知识创新方向性项目资助。(生物谷Bioon.com)
生物谷推荐原文出处:
Cell Res. doi:10.1038/cr.2010.169
Aquaporin3 is a sperm water channel essential for postcopulatory sperm osmoadaptation and migration.
Chen Q, Peng H, Lei L, Zhang Y, Kuang H, Cao Y, Shi QX, Ma T, Duan E.
[1] State Key Laboratory of Reproductive Biology, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China [2] Graduate School of Chinese Academy of Sciences, Beijing 100049, China.
Abstract
In the journey from the male to female reproductive tract, mammalian sperm experience a natural osmotic decrease (e.g., in mouse, from ~415 mOsm in the cauda epididymis to ~310 mOsm in the uterine cavity). Sperm have evolved to utilize this hypotonic exposure for motility activation, meanwhile efficiently silence the negative impact of hypotonic cell swelling. Previous physiological and pharmacological studies have shown that ion channel-controlled water influx/efflux is actively involved in the process of sperm volume regulation; however, no specific sperm proteins have been found responsible for this rapid osmoadaptation. Here, we report that aquaporin3 (AQP3) is a sperm water channel in mice and humans. Aqp3-deficient sperm show normal motility activation in response to hypotonicity but display increased vulnerability to hypotonic cell swelling, characterized by increased tail bending after entering uterus. The sperm defect is a result of impaired sperm volume regulation and progressive cell swelling in response to physiological hypotonic stress during male-female reproductive tract transition. Time-lapse imaging revealed that the cell volume expansion begins at cytoplasmic droplet, forcing the tail to angulate and form a hairpin-like structure due to mechanical membrane stretch. The tail deformation hampered sperm migration into oviduct, resulting in impaired fertilization and reduced male fertility. These data suggest AQP3 as an essential membrane pathway for sperm regulatory volume decrease (RVD) that balances the "trade-off" between sperm motility and cell swelling upon physiological hypotonicity, thereby optimizing postcopulatory sperm behavior.







