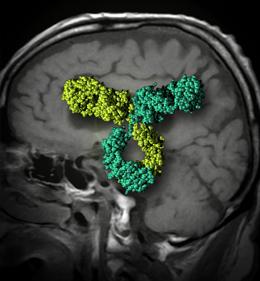
Antibodies that bind to more than one target can get past the blood–brain barrier.
By defying the classical rules of antibody engineering, researchers have constructed an antibody that is readily shuttled into the brain. The results suggest that the approach could be used to generate antibody-based therapies for brain diseases.
Antibodies — proteins used by the immune system to neutralize damaging foreign substances — are prized both in nature and in the laboratory because they are highly specific. But researchers in academia and industry are increasingly striving to make antibodies that bind to more than one target.
"We're about to see a wave of bispecific antibodies," says Ryan Watts, a neurobiologist at Genentech, a biotechnology firm based in San Francisco, California, that pioneered the development of therapeutic antibodies and is now a division of the Swiss pharmaceutical giant, Roche. "They are the hot topic in the field."
But few of those antibodies will be able to penetrate the shield of tightly packed cells known as the blood–brain barrier, which protects the brain from blood-borne intruders such as bacteria, but also keeps out most large drug molecules. Antibody concentrations in the brain are typically about a thousand times lower than in the blood, says Watts.
In two papers published today in Science Translational Medicine1,2, Watts and his collaborators report the design of an antibody that surmounts this obstacle.
The antibody targets two proteins. The first, called β-secretase 1, is a popular target for drugs to treat Alzheimer's disease, because it has an important role in the production of amyloid peptides in the brain. According to the 'amyloid hypothesis', amyloid aggregates are responsible for the hallmark brain damage and memory loss of the condition.
The second protein targeted by the antibody is the transferrin receptor, which activates a molecular channel that normally imports iron into the brain. By clinging to this receptor, the antibody is transported into the brain, where it can act against β-secretase 1.
The double-duty antibody performed well in mouse models of Alzheimer's disease: a day after receiving a single injection of the antibody, concentrations of amyloid-β in the brain plummeted by 47%1.
But before the Genentech team could achieve those results, they had to defy another cardinal rule of antibody engineering.
Letting go
The strength of the interaction between an antibody and its target is called its affinity: the higher the affinity, the stronger the interaction. Most antibody engineers strive to make antibodies with the highest affinity and tightest binding possible.
Watts and Mark Dennis, an antibody engineer at Genentech, started out making antibodies with high affinity against the transferrin receptor, but found that they stayed locked within the blood vessels of the brain rather than penetrating its tissues. Dennis reasoned that the receptor could be the trapping the antibody in the vasculature, so he engineered an antibody with lower affinity for it.
As he had predicted, the lower-affinity antibody was distributed more broadly in the brain2. Watts and Dennis liken the transportation of molecules by the receptor to a ski lift. "The high-affinity antibodies never get off the ski lift," says Watts. "The low-affinity antibodies get off and are widely distributed."
"It's a nice demonstration of how, when you look at multispecific protein therapeutics, you have to throw out some of the rules and paradigms that we learned through monoclonal antibodies," says David Hilbert, head of research at Zyngenia in Rockville, Maryland. His company is developing multispecific antibodies that have been modified to recognize as many as five different targets.
Hilbert notes that low-affinity, multispecific antibody designs might also be useful in other arenas. Cancer stem cells, for example, are often identified on the basis of a combination of marker proteins that they express on their surfaces. But these markers are sometimes present in other combinations on healthy cells. A traditional high-affinity monoclonal antibody could kill those healthy cells as well as their cancer targets, but a low-affinity, multi-targeted antibody could more selectively target the cancer stem cells, says Hilbert.
"Such a drug would tumble through the host transiently binding cells that express only one or two of the targets," says Hilbert. "But when the drug encounters a cancer stem cell, it will engage all targets, thereby turning multiple low-affinity interactions into a net high-affinity interaction."
Competitive edge
Not everyone is a fan of the low-affinity approach, however. "From a technical standpoint, it's beautiful work," says William Pardridge, an endocrinologist at the University of California, Los Angeles, who has long studied the blood–brain barrier. "But conceptually, I think they've gone down a blind alley."
Pardridge is the founder of a competing company, ArmaGen in Santa Monica, California. He says that his lab and the scientists at ArmaGen have dispersed their antibodies throughout the brain using the same receptor, without having to reduce affinity. He adds that Genentech's design has left the company with an antibody that must be given at unreasonably high doses. "I hope it goes down this path," Pardrige says. "Then I'll have one less competitor."
Watts counters that the doses used in Genentech's mouse study are normal. He says that it is common for therapeutic doses of antibodies to be higher in mouse models than in humans, where the antibodies tend to remain active for longer before being broken down by the body. Genentech intends to move forward with the platform, says Watts. "This was a critical proof-of-concept. We're looking at applying it to other targets in the central nervous system.