12月16日,国际著名病毒学期刊Journal of Virology在线发表了中科院上海巴斯德研究所钟劲研究组的最新成果,该研究主要阐明了一种新型的丙型肝炎病毒细胞感染模型的建立。
丙型肝炎病毒(hepatitis C virus, HCV)主要经过血液传播,能导致急性和慢性肝炎,肝硬化以及肝癌。目前全球约有1.7亿HCV感染者,我国普通人群感染者占全球HCV感染人数约25%,属于丙肝高发区。目前世界上还没有可预防HCV感染的疫苗和抗病毒药物,目前的治疗手段疗效有限、副作用较高,因此HCV感染对我国的公众健康构成了严重的威胁,HCV疫苗和创新抗病毒药物的研制对于提高和保障我国国民身体健康将有着极其深远的意义。
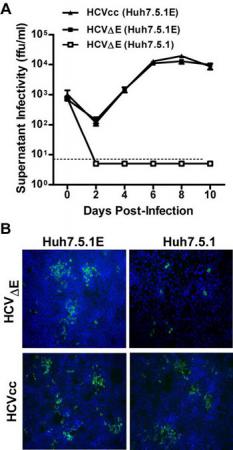
HCV体外细胞感染模型的缺乏是阻碍HCV 分子生物学和免疫学研究的主要因素。博士研究生李瑞在钟劲研究员的指导下构建了一个能产生只具有一次性感染力的类病毒颗粒的HCV细胞模型。该系统基于反式互补的原理:利用反式表达的同源病毒或者水疱性口炎病毒的包膜蛋白来包装缺失包膜蛋白序列的HCV基因组RNA。这种具有一次性感染力的HCV在生物学特性上可以真实模拟真病毒入侵和复制的过程,既能弥补现有系统在研究HCV生命周期中的不足,也为新型HCV疫苗的开发提供了新的思路。
巴斯德所周保罗研究组参与了该工作。此项研究得到了国家‘973’计划项目、“十一五”传染病防治科技重大专项、国家自然科学基金、以及法国TOTAL研究基金等资助。
原文出处:
J. Virol. doi:10.1128/JVI.02313-10
Production of hepatitis C virus lacking the envelope-encoding genes for single-cycle infection by providing homologous envelope proteins or vesicular stomatitis virus glycoproteins in trans
Rui Li, Yan Qin, Ying He, Wanyin Tao, Nan Zhang, Cheguo Tsai, Paul Zhou, and Jin Zhong*
Unit of Viral Hepatitis, Unit of Antiviral immunity and genetic therapy, Key Laboratory of Molecular Virology and Immunology, Institut Pasteur of Shanghai, Shanghai Institutes for Biological Sciences, Chinese Academy of Science, Shanghai, 200025, China
Abstract
Hepatitis C virus (HCV) infection is a major worldwide health problem. The envelope glycoproteins are the major components of viral particles. Here we developed a trans-complementation system that allows the production of infectious HCV particles containing the envelope protein-encoding regions deleted genome (HCVE). The lack of envelope proteins could be efficiently complemented by the expression of homologous envelope proteins in trans. HCVE production could be enhanced significantly by previously described adaptive mutations in NS3 and NS5A. Moreover, HCVE could be propagated and passaged in the packaging cells stably expressing HCV envelope proteins while only resulted in single-round infection in wild-type cells. Interestingly, we found that vesicular stomatitis virus (VSV) glycoproteins could efficiently rescue the production of HCV lacking endogenous envelope proteins, which no longer required apolipoprotein E for virus production. The VSV glycoprotein-mediated viral entry could allow for the bypass of natural HCV entry process and the delivery of HCV replicon RNA into HCV receptor deficient cells. Our development provided a new tool to produce single-cycle infectious HCV particles which should be useful for studying individual steps of HCV life cycle, and may also provide a new strategy for HCV vaccine development.







