来自南开大学和中科院上海生命科学研究院的研究人员开发了一项创新的iPS技术,在山中伸弥经典方法的基础上添加独特的因子Zscan4,证实可以促进重编程过程中的基因组稳定,显著提高生成的iPS细胞质量。相关结果发表在11月13日的《细胞研究》(Cell research)杂志上。
来自南开大学生命科学学院的刘林(Lin Liu)教授和中科院上海生命科学研究院的李劲松(Jinsong Li)研究员是这篇论文的共同通讯作者。前者主要在发育生物学和生殖生物技术领域,尤其是在最终能造福人类健康的胚胎工程和再生医学领域,进行基础科学及生物医学应用方面的研究。后者的主要研究方向是体细胞重编程与诱导多能干细胞。
2006年,日本京都大学山中伸弥(Shinya Yamanaka)团队通过向人体皮肤成纤维细胞中植入4个经过重新编码的基因Oct3/4, Sox2, c-Myc, Klf4 ,将成纤维细胞重新编排变成了全能性的类胚胎干细胞。他们将这种 ”返老还童”的重新编排细胞称之为”诱导式多能性干细胞”,即iPS细胞。此项技术不仅为发展再生医疗开创了道路,也对整个医学研究的发展做出巨大贡献。
然而由于其潜在的致癌性和重编程效率低限制了iPS细胞在细胞治疗中的潜在应用。尽管近年来研究人员开发了大量的创新技术使得iPS细胞生成效率大为提高。然而一些研究也表明iPS细胞存在着异常的基因表达以及基因组异常,iPS细胞临床应用的安全性成为了人们热点关注的问题。四倍体互补分析(TCA)实验证实大部分小鼠iPS细胞也并非具有完全多能性,无法生成活体小鼠。这些可能与四种因子诱导重编程早期发生的DNA损伤反应(DDR)有关。与之相反,核移植可以高效准确地将体细胞重编程为胚胎干细胞,并生成活体全胚胎干细胞小鼠。
由此,研究人员推测参与卵母细胞诱导重编程的因子或许能够对重编程过程中的体细胞基因组起稳定作用,提高生成的iPS细胞的质量。为了验证这一假设,研究人员筛查了在iPS细胞诱导过程中能够减少DDR信号的因子,发现了在合子基因组激活阶段高表达的独特基因Zscan4。
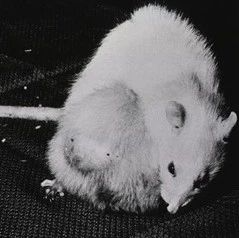
随后研究人员采用Zscan4结合四种因子诱导细胞重编程,证实不仅可以显著地减少DDR,还可以大大提高iPS细胞生成效率。加入Zscan4稳定了基因组DNA,导致了p53下调。此外,在病毒感染后3天,Zscan4就可以通过一种端粒重组机制来促进端粒延长。因此添加Zscan4生成的iPS细胞相比于经典的iPS细胞显示更长端粒。引人注目的是,利用四倍体互补实验证实通过这种“Zscan4实验方案”生成的iPS细胞系超过50%的生成了出生存活(live-borne)的全iPS细胞小鼠,而采用山中伸弥经典的方法只有1/12。
在这篇文章中,研究人员提出了一种Zscan4结合四个因子的创新iPS技术,不仅可以显著提高重编程效率,还可以大大提高iPS细胞的质量。此外,新研究发现还第一证实了参与卵母细胞介导重编程的因子可以帮助维持重编程过程中的基因组稳定,由此促进高质量的iPS细胞生成。

 Zscan4 promotes genomic stability during reprogramming and dramatically improves the quality of iPS cells as demonstrated by tetraploid complementation
Zscan4 promotes genomic stability during reprogramming and dramatically improves the quality of iPS cells as demonstrated by tetraploid complementation
Jing Jiang, Wenjian Lv, Xiaoying Ye, Lingbo Wang, Man Zhang, Hui Yang, Maja Okuka, Chikai Zhou, Xuan Zhang, Lin Liu and Jinsong Li
Induced pluripotent stem (iPS) cells generated using Yamanaka factors have great potential for use in autologous cell therapy. However, genomic abnormalities exist in human iPS cells, and most mouse iPS cells are not fully pluripotent, as evaluated by the tetraploid complementation assay (TCA); this is most likely associated with the DNA damage response (DDR) occurred in early reprogramming induced by Yamanaka factors. In contrast, nuclear transfer can faithfully reprogram somatic cells into embryonic stem (ES) cells that satisfy the TCA. We thus hypothesized that factors involved in oocyte-induced reprogramming may stabilize the somatic genome during reprogramming, and improve the quality of the resultant iPS cells. To test this hypothesis, we screened for factors that could decrease DDR signals during iPS cell induction. We determined that Zscan4, in combination with the Yamanaka factors, not only remarkably reduced the DDR but also markedly promoted the efficiency of iPS cell generation. The inclusion of Zscan4 stabilized the genomic DNA, resulting in p53 downregulation. Furthermore, Zscan4 also enhanced telomere lengthening as early as 3 days post-infection through a telomere recombination-based mechanism. As a result, iPS cells generated with addition of Zscan4 exhibited longer telomeres than classical iPS cells. Strikingly, more than 50% of iPS cell lines (11/19) produced via this “Zscan4 protocol” gave rise to live-borne all-iPS cell mice as determined by TCA, compared to 1/12 for lines produced using the classical Yamanaka factors. Our findings provide the first demonstration that maintaining genomic stability during reprogramming promotes the generation of high quality iPS cells.