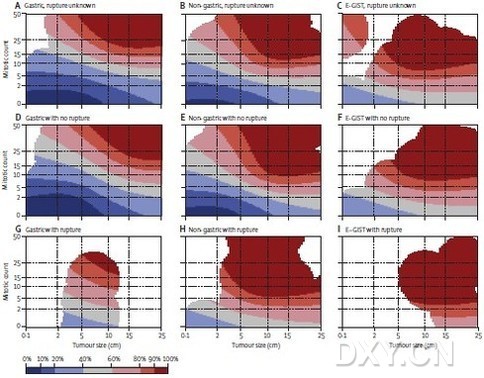
研究人员开发出了预后等高线图,这些预后热图在肿瘤学上是一个新概念,可以与基因表达热图进行比较。每个肿瘤的风险用颜色等高线来描绘,红颜色意味着高风险,蓝色意味着低风险。
对于经诊断可行手术的GIST患者,术后若考虑辅助性药物治疗时,评估手术的复发风险显得尤为重要。近日,来自芬兰赫尔辛基大学中心医院的芬兰研究人员已经开发出一种用来评估胃肠道间质瘤(GIST)患者复发风险的新方法。他们的研究成果在线刊登于国际著名杂志The Lancet Oncology上,有望更准确地筛选出那些有GIST复发风险和最可能从辅助全身治疗中受益的患者。
在同期述评中,本研究第一作者Heikki Joensuu博士说道,依据在2011年美国临床肿瘤学会(ASCAO)上进行的报告,辅助伊马替尼辅助治疗的标准周期现在延长至3年,然而3年的伊马替尼辅助治疗不仅与无复发生存率和总生存率改善有关,还与不良反应有关。因此,确定哪些GIST病人需要辅助治疗是一个关键性的挑战。目前,许多GIST病人都给予伊马替尼治疗,即使有些病人只通过手术就可以治愈。这篇文章提供了非常重要的数据,使用新建立的预测模型,给予临床医生一个坚实的理论方法去区别高风险易复发的亚群。这项个体化的方法将会降低成本和副作用,将重点关注到那些需要辅助治疗的病人身上。
本研究中,研究人员汇集了基于人群队列的个体患者信息,以获得2560例未接受辅助治疗而手术可行的GIST患者数据库。采用3种常用的复发预测模型去对肿瘤复发风险进行分层:国立卫生研究所(NIH)共识标准,修订后的共识标准以及武装部病理研究所(AFIP)标准。同时他们还用比例风险非线性模型分析无复发生存率的预后因素。
手术后估计的15年无复发生存率为59.9%(95% CI 56.2—63.6),并且在随访的前十年,几乎无复发。主要不良预后因素有肿瘤偏大、有丝分裂计数高、非胃原发部位、肿瘤出现破裂和男性性别。研究人员通过受试者操作特征(ROC)曲线进行灵敏度分析,发现3个主要的风险分层模型估计GIST的10年无复发生存率都是相当准确的,NIH共识分类标准的AUC为0.79(95%CI0.76-0.81,P <0.0001),修改后的共识标准为0.78(95%CI 0.75-0.80, P <0.0001),AFIP标准为0.82(95%CI 0.80-0.85,P <0.0001),同时,修改后的共识标准识别出单个高风险类别。由于肿瘤的大小和有丝分裂计数与GIST复发风险之间存在非线性关系,非线性模型能准确预测出复发的风险,曲线下面积(AUC)为0.88(95%CI 0.86-0.90)。与传统的风险分层方法相比,从非线性模型而来的预后等高线图可提供最准确的预后估计。
因此,研究人员认为,修改后的共识标准在考虑辅助治疗的单个高风险类别方面非常有效,而非线性模型非常适合于个体患者咨询,尤其是那些处在复发风险边缘的病人。

Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts
Heikki Joensuu, Aki Vehtari DSci, Jaakko Riihimäki, Toshirou Nishida, Sonja E Steigen, Peter Brabec, Lukas Plank, Bengt Nilsson, Claudia Cirilli, Chiara Braconi Andrea Bordoni, Magnus K Magnusson, Zdenek Linke, Jozef Sufliarsky, Massimo Federico, Jon G Jonasson, Angelo Paolo Dei Tos, Piotr Rutkowski
Background: The risk of recurrence of gastrointestinal stromal tumour (GIST) after surgery needs to be estimated when considering adjuvant systemic therapy. We assessed prognostic factors of patients with operable GIST, to compare widely used risk-stratification schemes and to develop a new method for risk estimation.
Methods: Population-based cohorts of patients diagnosed with operable GIST, who were not given adjuvant therapy, were identified from the literature. Data from ten series and 2560 patients were pooled. Risk of tumour recurrence was stratified using the National Institute of Health (NIH) consensus criteria, the modified consensus criteria, and the Armed Forces Institute of Pathology (AFIP) criteria. Prognostic factors were examined using proportional hazards and non-linear models. The results were validated in an independent centre-based cohort consisting of 920 patients with GIST.
Findings: Estimated 15-year recurrence-free survival (RFS) after surgery was 59•9% (95% CI 56•2—63•6); few recurrences occurred after the first 10 years of follow-up. Large tumour size, high mitosis count, non-gastric location, presence of rupture, and male sex were independent adverse prognostic factors. In receiver operating characteristics curve analysis of 10-year RFS, the NIH consensus criteria, modified consensus criteria, and AFIP criteria resulted in an area under the curve (AUC) of 0•79 (95% CI 0•76—0•81), 0•78 (0•75—0•80), and 0•82 (0•80—0•85), respectively. The modified consensus criteria identified a single high-risk group. Since tumour size and mitosis count had a non-linear association with the risk of GIST recurrence, novel prognostic contour maps were generated using non-linear modelling of tumour size and mitosis count, and taking into account tumour site and rupture. The non-linear model accurately predicted the risk of recurrence (AUC 0•88, 0•86—0•90).
Interpretation: The risk-stratification schemes assessed identify patients who are likely to be cured by surgery alone. Although the modified NIH classification is the best criteria to identify a single high-risk group for consideration of adjuvant therapy, the prognostic contour maps resulting from non-linear modelling are appropriate for estimation of individualised outcomes.
Funding: Academy of Finland, Cancer Society of Finland, Sigrid Juselius Foundation and Helsinki University Research Funds.
文献链接:https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045%2811%2970299-6/abstract








