近期,上海应用物理研究所物理生物学实验室的樊春海与黄庆课题组合作发展了两种新型的纳米载运体系,将具有免疫刺激效用的CpG寡核苷酸药物偶联到纳米结构上,可以有效被哺乳动物免疫细胞摄取,并刺激后者产生免疫反应而释放细胞因子。相关论文已于近日发表于纳米领域的权威杂志《ACS Nano》。
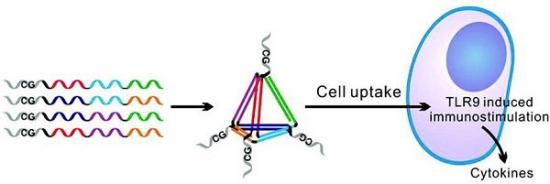
CpG寡核苷酸是指未经甲基化修饰的胞嘧啶-鸟嘌呤核酸序列,由于其可以通过TLR9信号通路有效引发哺乳动物免疫反应,因此可以作为一种良好的免疫佐剂用于抗感染和肿瘤等的辅助。然而,人工合成的CpG寡核苷酸通常难以进入细胞,因而如何寻找稳定、高活性和生物相容性好的载运体系是CpG药物应用的关键。纳米金粒子是一种生物相容的纳米材料,而且其表面可以负载大量的巯基修饰的寡核苷酸。研究表明,当CpG寡核苷酸负载到纳米金粒子表面后,可以很容易地被细胞内吞,并产生显著的免疫刺激作用。其刺激产生的TNF-alpha, IL-6, IL-12等细胞因子浓度较裸寡核苷酸提供了两个数量级,且显著高于商品化的载运试剂lipofectamin。
近年来,DNA纳米技术成为一个蓬勃发展的领域。相对于常用的无机纳米材料而言,DNA自组装纳米结构可以可控制备,且结构精确可调。研究人员在之前研究的一种四面体DNA纳米结构(Adv. Mater. 2010, 22, 4754; Anal. Chem. 2011, 83, 7418; Chem. Commun. 2011, 47, 6254)的基础上,将刚性的DNA四面体结构与CpG寡核苷酸结合,发现这种三维DNA纳米结构可以有效地将CpG寡核苷酸载运到细胞内,并且在胞浆内可以稳定存在8小时以上。
由于稳定性与摄取效率的同步提高,这种纳米载体极大增强了CpG寡核苷酸的免疫刺激效果。同时,DNA纳米结构本身就是核酸分子,容易与待载运核酸分子偶联,且在体内具有可降解、无免疫原性等优点,因而这种新型核酸载运体系显示了在疾病诊疗中的广泛应用前景。

Self-Assembled Multivalent DNA Nanostructures for Noninvasive Intracellular Delivery of Immunostimulatory CpG Oligonucleotides
Jiang Li, Hao Pei, Bing Zhu, Le Liang, Min Wei, Yao He, Nan Chen, Di Li, Qing Huang*, and Chunhai Fan*
Designed oligonucleotides can self-assemble into DNA nanostructures with well-defined structures and uniform sizes, which provide unprecedented opportunities for biosensing, molecular imaging, and drug delivery. In this work, we have developed functional, multivalent DNA nanostructures by appending unmethylated CpG motifs to three-dimensional DNA tetrahedra. These small-sized functional nanostructures are compact, mechanically stable, and noncytotoxic. We have demonstrated that DNA nanostructures are resistant to nuclease degradation and remain substantially intact in fetal bovine serum and in cells for at least several hours. Significantly, these functional nanostructures can noninvasively and efficiently enter macrophage-like RAW264.7 cells without the aid of transfection agents. After they are uptaken by cells, CpG motifs are recognized by the Toll-like receptor 9 (TLR9) that activates downstream pathways to induce immunostimulatory effects, producing high-level secretion of various pro-inflammatory cytokines including tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-12. We also show that multivalent CpG motifs greatly enhance the immunostimulatory effect of the nanostructures. Given the high efficacy of these functional nanostructures and their noncytotoxic nature, we expect that DNA nanostructures will become a promising tool for targeted drug delivery.
文献链接:https://pubs.acs.org/doi/abs/10.1021/nn202774x








